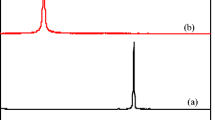
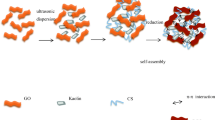
Abstract
Based on the industrialized graphene oxide (GO) product, poly(vinyl alcohol) (PVA)/GO nano-composite hydrogels were prepared through in situ crosslinking by incorporation of N-[(trimethoxysilyl)propyl] ethylenediamine-triacetic acid sodium (CSA) as a compatibilizer. Introduction of CSA led to more efficient grafting of PVA molecules onto GO surface with increasing average layer thickness through covalent and hydrogen bonding interaction, while GO was exfoliated and uniformly distributed in PVA matrix. Addition of appropriate content of GO can improve the storage modulus and the effective crosslinking density (υe) of the composite hydrogel, and the network structure with GO as crosslinking point formed, resulting in the remarkable increase of the hydraulic impact resistance, mechanical strength and toughness of the hydrogel. Pb2+ adsorption capacity of the hydrogel increased with GO content, while the adsorption belonged to the second-order kinetic model and fitted Langmuir adsorption isotherm model, indicating the homogeneous nature of monolayer chemical adsorption of Pb2+. A relatively good reusability of the composite hydrogel beads for Pb2+ removal can be achieved.










Similar content being viewed by others
References
Volesky B, Holan ZR (1995) Biosorption of heavy metals. Biotechnol Prog 11:235–250
Celik A, Demirba A (2005) Removal of heavy metal ions from aqueous solutions via adsorption onto modified lignin from pul** wastes. Energy Sources 27:1167–1177
Chan WC (1994) Removal of heavy metal ions with water-insoluble amphoteric sodium tertiary amine sulfonate starches. J Polym Res 1:221–226
Bereket G, Aroğuz AZ, Ozel MZ (1997) Removal of Pb (II), Cd (II), Cu (II), and Zn (II) from aqueous solutions by adsorption on bentonite. J. Colloid Interf. Sci. 187:338–343
Gupta SS, Bhattacharyya KG (2005) Interaction of metal ions with clays: I. A case study with Pb (II). Appl. Clay Sci 30:199–208
Tunali S, Akar T, Özcan AS (2006) Equilibrium and kinetics of biosorption of lead (II) from aqueous solutions by Cephalosporium aphidicola. Sep Purif Technol 47:105–112
Nadeem M, Nadeem R, Nadeem HU (2005) Accumulation of lead and cadmium in different organs of chicken. Pak J Sci Res 57:71
Zeledón-Toruño Z, Lao-Luque C, Solé-Sardans M (2005) Nickel and copper removal from aqueous solution by an immature coal (leonardite): effect of pH, contact time and water hardness. J Chem Technol Biotechnol 80:649–656
Erdem E, Karapinar N, Donat R (2004) The removal of heavy metal cations by natural zeolites. J. Colloid Interf. Sci. 280:309–314
Apak R, Güçlü K, Turgut MH (1998) Modeling of copper (II), cadmium (II), and lead (II) adsorption on red mud. J. Colloid Interf. Sci. 203:122–130
Bulut Y, Zeki TEZ (2007) Removal of heavy metals from aqueous solution by sawdust adsorption. J Environ SCI-China 19:160–166
Hossain MA, Ngo HH, Guo WS (2012) Palm oil fruit shells as biosorbent for copper removal from water and wastewater: experiments and sorption models. Bioresour Technol 113:97–101
Parajuli D, Inoue K, Ohto K (2005) Adsorption of heavy metals on crosslinked lignocatechol: a modified lignin gel. React Funct Polym 62:129–139
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666–669
Sham AY, Notley SM (2013) A review of fundamental properties and applications of polymer-graphene hybrid materials. Soft Matter 9:6645–6653
Shamsi R, Koosha M, Mahyari M (2016) Improving the mechanical, thermal and electrical properties of polyurethane- graphene oxide nanocomposites synthesized by in-situ polymerization of ester-based polyol with hexamethylene diisocyanate. J Polym Res 23:262–272
Chen J, Li C, Shi G (2013) Graphene materials for electrochemical capacitors. The J Phys Chem Lett 4:1244–1253
Huang X, Qi X, Boey F, Zhang H (2012) Graphene-based composites. Chem Soc Rev 41:666–686
Li X, Zhou H, Wu W (2015) Studies of heavy metal ion adsorption on chitosan/Sulfydryl-functionalized graphene oxide composites. J Colloid Interf Sci 448:389–397
Jiao C, **ong J, Tao J (2016) Sodium alginate/graphene oxide aerogel with enhanced strength–toughness and its heavy metal adsorption study. J Biol Macromol 83:133–141
Ji Y, Ma J, Liang B (2005) In situ polymerization and in situ compatibilization of polymer blends of poly (2, 6-dimethyl-1, 4-phenylene oxide) and polyamide 6. Mater Lett 59:1997–2000
Szabó T, Berkesi O, Forgó P, Josepovits K, Sanakis Y, Petridis D, Dékány I (2006) Evolution of surface functional groups in a series of progressively oxidized graphite oxides. Chem Mater 18:2740–2749
Ferrari AC, Meyer JC, Scardaci V (2006) Raman spectrum of graphene and graphene layers. Phys Rev Lett 97:187401
Guo J, Ren L, Wang R, Zhang C, Yang Y, Liu T (2011) Water dispersible graphene noncovalently functionalized with tryptophan and its poly (vinyl alcohol) nanocomposite. Compos Part B-Eng 42:2130–2135
Paredes JI, Villar-Rodil S, Solís-Fernández P, Martínez-Alonso A, Tascon JMD (2009) Atomic force and scanning tunneling microscopy imaging of graphene nanosheets derived from graphite oxide. Langmuir 25:5957–5968
Huang T, Liu P, Lu RG, Huang ZY, Chen HM, Li TS (2012) Modification of polyetherimide by phenylethynyl terminated agent for improved tribological, macro-and micro-mechanical properties. Wear 292:25–32
Cao Y, Feng J, Wu P (2010) Preparation of organically dispersible graphene nanosheet powders through a lyophilization method and their poly (lactic acid) composites. Carbon 48:3834–3839
Cao Y, Lai Z, Feng J, Wu P (2011) Graphene oxide sheets covalently functionalized with block copolymers via click chemistry as reinforcing fillers. J Mater Chem 21:9271–9278
Huang T, Lu R, Su C, Wang H, Guo Z, Liu P, Huang Z, Chen H, Li T (2012) Chemically modified graphene/polyimide composite films based on utilization of covalent bonding and oriented distribution. ACS Appl Mater Inter 4:2699–2708
Meng Y, Ye L (2017) Synthesis and swelling property of the starch-based macroporous superabsorbent. J Appl Polym Sci 134:44855–44864
Meng Y, Ye L (2017) Synthesis and swelling property of superabsorbent starch grafted with acrylic acid/2-acrylamido-2-methyl-1-propanesulfonic acid. J Sci Food Agric 97:3831–3840
Zhang Y, Hui B, Ye L (2016) Preparation and structure of poly (vinyl alcohol)/Polyacrylate elastomer composite hydrogels and their application in wastewater treatment by immobilizing with microorganisms. Ind Eng Chem Res 55:9934–9943
Huang F, Zheng Y, Yang Y (2007) Study on macromolecular metal complexes: synthesis, characterization, and fluorescence properties of stoichiometric complexes for rare earth coordinated with poly (acrylic acid). J Appl Polym Sci 103:351–357
Al-qudah YHF, Mahmoud GA, Khalek MAA (2014) Radiation crosslinked poly (vinyl alcohol)/acrylic acid copolymer for removal of heavy metal ions from aqueous solutions. J Radiat Res Appl Sci 7:135–145
Acknowledgements
This work was financially supported by State Key Laboratory of Polymer Materials Engineering of China(Grant No. sklpme2016-2-07).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meng, Y., Zhang, G. & Ye, L. In situ crosslinking of poly(vinyl alcohol)/graphene oxide Nano-composite hydrogel: intercalation structure and adsorption mechanism for advanced Pb(II) removal. J Polym Res 25, 168 (2018). https://doi.org/10.1007/s10965-018-1569-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-018-1569-4