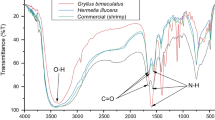
Abstract
Black Soldier Fly (BSF) is a novel option to convert organic waste into high economic products such as protein, lipid, and chitin. Due to the drawbacks of petrochemical-based plastic, the biodegradable film has attracted much attention; among these, chitin-based packaging film is a promising material. This study extracted the chitin from BSF using a co-solvent of glycerol and hydrochloric acid (HCl). The chitin nanofiber has a dimension of 34 nm in width and 494 in length following Transmission Electron Microscopy analysis (TEM). Besides, the obtained chitin was also added to the gelatin-based film for packaging application. The chitin/gelation packaging was evaluated for its suitability in food application by testing its antioxidant activity, thickness, grammage, opacity, moisture, and water solubility. The film developed with more than 0.5 wt.% chitin and nanochitin showed high antioxidant activity, while adding chitin does not significantly change the thermal stability of the gelatin films. The chemical structure of chitin and bio-packaging was determined by Fourier Transform Infrared (FTIR), X-Ray Diffraction (XRD), and Thermogravimetric Analysis (TGA). This study provides a green and facile approach for chitin production from BSF by using co-solvent and reveals the potential of insect chitin in creating active bio-packaging.
Graphical Abstract







Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Le TM, Tran UPN, Duong YHP et al (2022) Development of a paddy-based biorefinery approach toward improvement of biomass utilization for more bioproducts. Chemosphere 289:133249
Liu C, Yao H, Chapman SJ et al (2020) Changes in gut bacterial communities and the incidence of antibiotic resistance genes during degradation of antibiotics by black soldier fly larvae. Environ Int 142:105834
Mertenat A, Diener S, Zurbrügg C (2019) Black Soldier Fly biowaste treatment–assessment of global warming potential. Waste Manag 84:173–181
Lalander C, Diener S, Zurbrügg C, Vinnerås B (2019) Effects of feedstock on larval development and process efficiency in waste treatment with black soldier fly (Hermetia illucens). J Clean Prod 208:211–219
Verheyen GR, Ooms T, Vogels L et al (2018) Insects as an alternative source for the production of fats for cosmetics. J Cosmet Sci 69:187–202
Smets R, Verbinnen B, Van De Voorde I et al (2020) Sequential extraction and characterisation of lipids, proteins, and chitin from black soldier fly (Hermetia illucens) larvae, prepupae, and pupae. Waste Biomass Valoriz 11:6455–6466
Hahn T, Tafi E, Paul A et al (2020) Current state of chitin purification and chitosan production from insects. J Chem Technol Biotechnol 95:2775–2795
Lin Y-S, Liang S-H, Lai W-L et al (2021) Sustainable extraction of chitin from spent pupal shell of black soldier fly. Processes 9:976
Wang H, ur Rehman K, Feng W et al (2020) Physicochemical structure of chitin in the develo** stages of black soldier fly. Int J Biol Macromol 149:901–907
Caligiani A, Marseglia A, Leni G et al (2018) Composition of black soldier fly prepupae and systematic approaches for extraction and fractionation of proteins, lipids and chitin. Food Res Int 105:812–820
Hajji S, Ghorbel-Bellaaj O, Younes I et al (2015) Chitin extraction from crab shells by Bacillus bacteria. Biological activities of fermented crab supernatants. Int J Biol Macromol 79:167–173
Qin Y, Lu X, Sun N, Rogers RD (2010) Dissolution or extraction of crustacean shells using ionic liquids to obtain high molecular weight purified chitin and direct production of chitin films and fibers. Green Chem 12:968–971
Hong S, Yuan Y, Yang Q et al (2018) Versatile acid base sustainable solvent for fast extraction of various molecular weight chitin from lobster shell. Carbohydr Polym 201:211–217
Hong S, Yang Q, Yuan Y et al (2019) Sustainable co-solvent induced one step extraction of low molecular weight chitin with high purity from raw lobster shell. Carbohydr Polym 205:236–243. https://doi.org/10.1016/j.carbpol.2018.10.045
Perosa A, Tundo P (2005) Selective hydrogenolysis of glycerol with raney nickel. Ind Eng Chem Res 44:8535–8537
Wolfson A, Dlugy C, Shotland Y (2007) Glycerol as a green solvent for high product yields and selectivities. Environ Chem Lett 5:67–71
Kaya M, Sofi K, Sargin I, Mujtaba M (2016) Changes in physicochemical properties of chitin at developmental stages (larvae, pupa and adult) of Vespa crabro (wasp). Carbohydr Polym 145:64–70
Waśko A, Bulak P, Polak-Berecka M et al (2016) The first report of the physicochemical structure of chitin isolated from Hermetia illucens. Int J Biol Macromol 92:316–320
Cheng H, Chen L, McClements DJ et al (2021) Starch-based biodegradable packaging materials: a review of their preparation, characterization and diverse applications in the food industry. Trends Food Sci Technol 114:70–82
De Clercq K, Schelfhout C, Bracke M et al (2016) Genipin-crosslinked gelatin microspheres as a strategy to prevent postsurgical peritoneal adhesions: in vitro and in vivo characterization. Biomaterials 96:33–46
Sahraee S, Milani JM, Ghanbarzadeh B, Hamishehkar H (2017) Physicochemical and antifungal properties of bio-nanocomposite film based on gelatin-chitin nanoparticles. Int J Biol Macromol 97:373–381
Said NS, Sarbon NM (2020) Response surface methodology (RSM) of chicken skin gelatin based composite films with rice starch and curcumin incorporation. Polym Test 81:106161
Soetemans L, Uyttebroek M, Bastiaens L (2020) Characteristics of chitin extracted from black soldier fly in different life stages. Int J Biol Macromol 165:3206–3214
Cd MD, Joseph R, Begum PMS et al (2020) Chitin nanowhiskers from shrimp shell waste as green filler in acrylonitrile-butadiene rubber: processing and performance properties. Carbohydr Polym 245:116505
Zadeh EM, O’Keefe SF, Kim Y-T (2018) Utilization of lignin in biopolymeric packaging films. ACS Omega 3:7388–7398
Fernández-Marín R, Fernandes SCM, Sánchez MÁA, Labidi J (2022) Halochromic and antioxidant capacity of smart films of chitosan/chitin nanocrystals with curcuma oil and anthocyanins. Food Hydrocoll 123:107119
Hu X, Yuan L, Han L et al (2019) Characterization of antioxidant and antibacterial gelatin films incorporated with Ginkgo biloba extract. RSC Adv 9:27449–27454
D’Hondt E, Soetemans L, Bastiaens L et al (2020) Simplified determination of the content and average degree of acetylation of chitin in crude black soldier fly larvae samples. Carbohydr Res 488:107899
Huang W-C, Zhao D, Guo N et al (2018) Green and facile production of chitin from crustacean shells using a natural deep eutectic solvent. J Agric Food Chem 66:11897–11901
Kaya M, Mujtaba M, Ehrlich H et al (2017) On chemistry of γ-chitin. Carbohydr Polym 176:177–186
Vinodh R, Sasikumar Y, Kim H-J et al (2021) Chitin and chitosan based biopolymer derived electrode materials for supercapacitor applications: a critical review. J Ind Eng Chem 104:155–171
Namboodiri MMT, Pakshirajan K (2020) Valorization of waste biomass for chitin and chitosan production. Waste biorefinery. Elsevier, Amsterdam, pp 241–266
Wang J, Chen C (2014) Chitosan-based biosorbents: modification and application for biosorption of heavy metals and radionuclides. Bioresour Technol 160:129–141
Etxabide A, Kilmartin PA, Maté JI, Gómez-Estaca J (2022) Characterization of glucose-crosslinked gelatin films reinforced with chitin nanowhiskers for active packaging development. LWT 154:112833
Al Sagheer FA, Al-Sughayer MA, Muslim S, Elsabee MZ (2009) Extraction and characterization of chitin and chitosan from marine sources in Arabian Gulf. Carbohydr Polym 77:410–419
Zozo B, Wicht MM, Mshayisa VV, van Wyk J (2022) The nutritional quality and structural analysis of black soldier fly larvae flour before and after defatting. Insects 13:168
Liao J, Huang H (2022) Preparation, characterization and gelation of a fungal nano chitin derived from Hericium erinaceus residue. Polymers (Basel) 14:474
Jang MK, Kong BG, Il JY et al (2004) Physicochemical characterization of α-chitin, β-chitin, and γ-chitin separated from natural resources. J Polym Sci Part A 42:3423–3432. https://doi.org/10.1002/pola.20176
Poerio A, Petit C, Jehl J-P et al (2020) Extraction and physicochemical characterization of chitin from cicada orni sloughs of the south-eastern French Mediterranean basin. Molecules 25:2543
Joseph B, Mavelil Sam R, Balakrishnan P et al (2020) Extraction of nanochitin from marine resources and fabrication of polymer nanocomposites: recent advances. Polymers (Basel) 12:1664
Gond RK, Gupta MK, Jawaid M (2021) Extraction of nanocellulose from sugarcane bagasse and its characterization for potential applications. Polym Compos 42:5400–5412
Huang C, Feng W, **ong J et al (2019) Impact of drying method on the nutritional value of the edible insect protein from black soldier fly (Hermetia illucens L.) larvae: amino acid composition, nutritional value evaluation, in vitro digestibility, and thermal properties. Eur Food Res Technol 245:11–21
Złotko K, Waśko A, Kamiński DM et al (2021) Isolation of chitin from black soldier fly (Hermetia illucens) and its usage to metal sorption. Polymers (Basel) 13:818
Pulla-Huillca PV, Gomes A, Quinta Barbosa Bittante AM et al (2021) Wettability of gelatin-based films: the effects of hydrophilic or hydrophobic plasticizers and nanoparticle loads. J Food Eng 297:110480. https://doi.org/10.1016/j.jfoodeng.2021.110480
Hanani ZAN, Roos YH, Kerry JP (2014) Use and application of gelatin as potential biodegradable packaging materials for food products. Int J Biol Macromol 71:94–102
Li Y, Cao C, Pei Y et al (2019) Preparation and properties of microfibrillated chitin/gelatin composites. Int J Biol Macromol 130:715–719
Liu F, Chiou B-S, Avena-Bustillos RJ et al (2017) Study of combined effects of glycerol and transglutaminase on properties of gelatin films. Food Hydrocoll 65:1–9
Rosseto M, Rigueto CVT, Krein DDC et al (2021) Accelerated aging of starch-gelatin films with enzymatic treatment. J Polym Environ 29:1063–1075
Roy S, Biswas D, Rhim J-W (2022) Gelatin/cellulose nanofiber-based functional nanocomposite film incorporated with zinc oxide nanoparticles. J Compos Sci 6:223
Hafsa J, Smach MA, Charfeddine B et al (2016) Antioxidant and antimicrobial proprieties of chitin and chitosan extracted from Parapenaeus Longirostris shrimp shell waste. Annales pharmaceutiques francaises. Elsevier, Amsterdam, pp 27–33
Ilyas HN, Zia KM, Rehman S et al (2021) Utilization of shellfish industrial waste for isolation, purification, and characterizations of chitin from crustacean’s sources in Pakistan. J Polym Environ 29:2337–2348
Acknowledgements
We acknowledge the support of time and facilities from Ho Chi Minh City University of Technology (HCMUT), VNU-HCM for this study.
Author information
Authors and Affiliations
Contributions
Conceptualization, formal analysis, investigation, writing, review and editing: TML, CLT, TXN; Writing—review and editing, investigation: YHPD; supervision, methodology, and conceptualization, review and editing: PKL; Funding acquisition, supervision, methodology, and conceptualization, review and editing: VTT. TML, TXN, and CLT have contributed equally and have the right to list their name first in their CV. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Le, T.M., Tran, C.L., Nguyen, T.X. et al. Green Preparation of Chitin and Nanochitin from Black Soldier Fly for Production of Biodegradable Packaging Material. J Polym Environ 31, 3094–3105 (2023). https://doi.org/10.1007/s10924-023-02793-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-023-02793-2