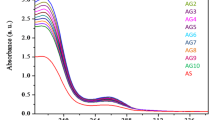
Abstract
Aspirin is a commonly used nonsteroidal anti-inflammatory drug, associated with many adverse effects. The adverse effects of aspirin such as tinnitus, Reye’s syndrome and gastrointestinal bleeding are caused due to conversion of aspirin into its active metabolite salicylic acid after oral intake. Glutathione is a naturally occurring antioxidant produced by the liver and nerve cells in the central nervous system. It helps to metabolize toxins, break down free radicles, and support immune function. This study aims to investigate and explore the possibility of inhibiting aspirin to salicylic acid conversion in presence of glutathione at a molecular level using spectroscopic techniques such as UV–Visible absorption, time-Resolved and time-dependent fluorescence and theoretical DFT/ TD-DFT calculations. The results of steady state fluorescence spectroscopy and time-dependent fluorescence indicated that the aspirin to salicylic acid conversion is considerably inhibited in presence of glutathione. Further, the results presented here might have significant clinical implications for individuals with variations in glutathione level.













Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Singh R, Tiwari MK, Gangopadhyay D, Mishra PC, Mishra H, Srivastava A, Singh RK (2018) Detection and monitoring of in vitro formation of salicylic acid from aspirin using fluorescence spectroscopic technique and DFT calculations. J Photochem Photobiol B Biol 189:292–297. https://doi.org/10.1016/j.jphotobiol.2018.11.004
Adebayo GI, Williams J, Healy S (2007) Aspirin esterase activity — Evidence for skewed distribution in healthy volunteers. Eur J Intern Med 18:299–303. https://doi.org/10.1016/j.ejim.2006.12.004
Traynor K (2019) Pharmacists help put aspirin recommendations into practice. Am J Health Syst Pharm 76(18):1368. https://doi.org/10.1093/ajhp/zxz147
Lee TY, Hsu YC, Tseng HC, Yu SH, Lin JT, Wu MS, Wu CY (2019) Association of daily aspirin therapy with risk of hepatocellular carcinoma in patients with chronic hepatitis B. JAMA Intern Med 179(5):633–640. https://doi.org/10.1001/jamainternmed.2018.8342
Moreira AB, Dias ILT, Neto GO, Zagatto EAG, Kubota LT (2005) Direct determination of paracetamol in powdered pharmaceutical samples by fluorescence spectroscopy. Anal Chim Acta 539(1–2):257–261. https://doi.org/10.1016/j.aca.2005.03.012
Patil SM, Sataraddi SR, Bagoji AM, Pathan RM, Nandibewoor ST (2014) Electroanalysis lectrochemical behavior of graphene-based sensors on the redox mechanism of aspirin. Electroanalysis 26:831–839. https://doi.org/10.1002/elan.201300650
Sostres C, Lanas A (2011) Gastrointestinal effects of aspirin. Nat Rev Gastroenterol Hepatol 8(7):385–394. https://doi.org/10.1038/nrgastro.2011.97
Sekhar RV (2021) GlyNAC supplementation improves glutathione deficiency, oxidative stress, mitochondrial dysfunction, inflammation, aging hallmarks, metabolic defects, muscle strength, cognitive decline, and body composition: implications for healthy aging. J Nutr. https://doi.org/10.1093/jn/nxab309
Jahanban-Esfahlan A, Panahi-Azar V (2016) Interaction of glutathione with bovine serum albumin: Spectroscopy and molecular docking. Food Chem 202:426–431. https://doi.org/10.1016/j.foodchem.2016.02.026
Wu G, Fang YZ, Yang S, Lupton JR, Turner ND (2004) Glutathione metabolism and its implications for health. J Nutr 134(3):489–492. https://doi.org/10.1093/jn/134.3.489
Singh R, Tiwari MK, Singh RK (2024) Inhibition conversion of aspirin into salicylic acid in presence of glycine. J Fluoresc. https://doi.org/10.1007/s10895-024-03675-z. In press
Singh AK, Firdaus Z, Prakash P, Singh R, Nandy N, Bansal M, Singh RK, Srivastava A, Roy JK, Mishra B, Singh RK (2017) Curcumin quantum dots mediated degradation of bacterial biofilms. Front Microbiol. https://doi.org/10.3389/fmicb.2017.01517
Singh R, Rai SK, Tiwari MK, Mishra A, Tewari AK, Mishra PC, Singh RK (2017) An excellent stable fluorescent probe: Selective and sensitive detection of trace amounts of Hg +2 ions in natural source of water. Chem Phys Lett 676:39–45. https://doi.org/10.1016/j.cplett.2017.03.046
Singh R, Kashyap S, Singh V, Kayastha AM, Mishra H, Saxena PS, Srivastava A, Singh RK (2018) QPRTase modified N-doped carbon quantum dots: a fluorescent bioprobe for selective detection of neurotoxin quinolinic acid in human serum. Biosens Bioelectr 101:103–109. https://doi.org/10.1016/j.bios.2017.10.017
Singh R, Yaday A, Shekhar S, Azad R, Singh RK, Kayastha AM (2019) Nitrogen Doped Carbon Quantum Dots Modified by Lens culinaris β-Galactosidase as a Fluorescent Probe for Detection of Lactose. J Fluoresc 29(3). https://doi.org/10.1007/s10895-019-02430-z
Singh R, Singh RK (2020) Detection of malachite green in water using edge excited label free fluorescent probe NCQDs. J Fluoresc 30:1281–1285. https://doi.org/10.1007/s10895-020-02603-1
Singh R, Tiwari MK, Goutam J, Singh RK (2020) Spectroscopic studies of CDPy molecule in different protic and aprotic solvents and investigation of antioxidant property. J Fluoresc 30(6):1439–1446. https://doi.org/10.1007/s10895-020-02589-w
Sharma P, Gangopadhyay D, Mishra PC, Mishra H, Singh RK (2016) Detection of in vitro metabolite formation of leflunomide: a fluorescence dynamics and electronic structure study. J Med Chem 59(7):3418–3426. https://doi.org/10.1021/acs.jmedchem.6b00088
Esrafili MD, Nurazar R (2014) A density functional theory study on the adsorption and decomposition of methanol on B12N12 fullerene-like nanocage. Superlattices and Microstructures, Elsevier 67:54–60. https://doi.org/10.1016/j.spmi.2013.12.020
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) General atomic and molecular electronic structure system. J Comput Chem 14:1347–1363. https://doi.org/10.1002/jcc.540141112
Dong L, Si-Jia W, Bo G, Lei S, Guang-Yue L (2021) Theoretical study on the sensing mechanism of a coumarin-based fluorescent probe for biological thiols. Spectrochim Acta Part A Mol Biomol Spectrosc 248:119268. https://doi.org/10.1016/j.saa.2020.119268
Dong L, Lei S, Shu-Huan G, Yu-Heng W, Guang-Yue L, Can-Hua Z (2020) Twisted intramolecular charge transfer: A time-dependent density functional theory study on the sensing mechanism of a Schiff base sensor for fluoride. Chem Phys Lett 738:136894. https://doi.org/10.1016/j.cplett.2019.136894
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark MJ, Heyd J, Brothers EN, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, in. Gaussian Inc, Wallingford, CT, USA
Govindarajan M, Karabacak M (2012) Spectroscopic properties, NLO, HOMO-LUMO and NBO analysis of 2,5-Lutidine. Spectrochim Acta A Mol Biomol Spectrosc 96:421–435. https://doi.org/10.1016/j.saa.2012.05.067
Robinson JW, Frame EMS, Frame GM II (2004) Undergraduate Instrumental Analysis, 6th edn. CRC Press, Boca Raton
Fleming I (1976) Frontier Orbitals and Organic Chemical Reactions. Wiley, London
Asiri AM, Karabacak M, Kurt M, Alamry KA (2011) Synthesis, molecular conformation, vibrational and electronic transition, isometric chemical shift, polarizability and hyperpolarizability analysis of 3-(4-methoxy-phenyl)-2-(4-nitro-phenyl)-acrylonitrile: a combined experimental and theoretical analysis. Spectrochim Acta Part A Mol Biomol Spectrosc 82(1):444–455. https://doi.org/10.1016/j.saa.2011.07.076
Kosar B, Albayrak C (2011) Spectroscopic investigations and quantum chemical computational study of (E)-4-methoxy-2-[(p-tolylimino)methyl]phenol. Spectrochim Acta A Mol Biomol Spectrosc 78(1):160–167. https://doi.org/10.1016/j.saa.2010.09.016
Janeoo S, Saroa A, Rakesh Kumar R, Kaur H (2022) Computational investigation of bioactive 2,3-diaryl quinolines using DFT method: FT- IR, NMR spectra, NBO, NLO, HOMO-LUMO transitions, and quantum-chemical properties. J Mol Struct 1253:132285, ISSN 0022–2860. https://doi.org/10.1016/j.molstruc.2021.132285
Rocha FS, Gomes AJ, Lunardi CN, Kaliaguine S, Patience GS (2018) Experimental methods in chemical engineering: ultraviolet visible spectroscopy—UV-Vis. Can J Chem Eng. https://doi.org/10.1002/cjce.23344
Lakowicz JR (2006) Principles of fluorescence spectroscopy. Plenum Press, New York and London Third Edition, pp 58–59
Miles CI, Schenk GH (1970) Fluorescence of Acetylsalicylic Acid in Solution and Its Measurement in Presence of Salicylic Acid. Anal Chem 42(6):656–659. https://doi.org/10.1021/ac60288a032
Bo G, Bo-Yu L, Shou-Liang Y, Yue-Hua L, Guang-Yue L (2021) A time-dependent density functional theory study of a fluorescent probe to detect hydroxyl radicals: Inhibiting the twisted intramolecular charge-transfer process. Spectrochim Acta Part A Mol Biomol Spectrosc 260:119928. https://doi.org/10.1016/j.saa.2021.119928
Gupta RC, Dwivedi SK, Ali R, Razi SS, Tiwari R, Krishnamoorthi S, Misra A (2020) A sensitive TICT Probe exhibiting ratiometric fluorescence repose to detect hydrazine in solution and gas phase. Spectrochimica Acta Part A 232:118153. https://doi.org/10.1016/j.saa.2020.11815
Li Y, Chu TS (2017) DFT/TDDFT Study on the Sensing Mechanism of a Fluorescent Probe for Hydrogen Sulfide: Excited State Intramolecular Proton Transfer Coupled Twisted Intramolecular Charge Transfer. J Phys Chem A 121(28):5245–5256. https://doi.org/10.1021/acs.jpca.7b02606
Wang C, Qiao Q, Chi W, Chen J, Liu W, Tan D, McKechnie S, Lyu DA, Jiang X-F, Zhou W, Xu N, Zhang Q, Xu Z, Liu X (2020) Quantitative design of bright fluorophores and AIEgens by the accurate prediction of twisted intramolecular charge transfer. J German Chem Soc 59:10160–10172. https://doi.org/10.1002/anie.201916357
Sasaki S, Drummen GPC, Konishi G (2016) Recent advances in twisted intramolecular charge transfer (TICT) fluorescence and related phenomena in materials chemistry. Journal of materials chemistry C 4:2731–2743. https://doi.org/10.1039/C5TC03933A
Lv X, Gao C, Han T, Shi H, Guo W (2020) Improving the quantum yields of fluorophores by inhibiting twisted intramolecular charge transfer using electron-withdrawing group-functionalized piperidine auxochromes, Royal society of chemistry. Chem Commun 56:715–718. https://doi.org/10.1039/C9CC09138F
Grabowski ZR, Rotkiewicz K, Rettig W (2003) Structural changes accompanying intramolecular electron transfer: focus on twisted intramolecular charge-transfer states and structures. Chem Rev 103(10):3899–4032. https://doi.org/10.1021/cr940745l
Georgieva I, Aquino AJA, Plasser F, Trendafilova N, Köhn A (2015) Intramolecular Charge-Transfer Excited-State Processes in 4-(N, N-Dimethylamino)benzonitrile: The Role of Twisting and the πσ* State. J Phys Chem A 119(24):6232–6243. https://doi.org/10.1021/acs.jpca.5b03282
Denisov GS, Golubev NS, Schreiber VM, Shajakhmedov ShS, Shurukhina AV (1997) Effect of intermolecular hydrogen bonding and proton transfer on fluorescence of salicylic acid. Journal of Molecular Structure, Volumes 436–437:153–160. https://doi.org/10.1016/S0022-2860(97)00136-1
Han C, Liu Y, Yang Y, Ni X, Lu J, Luo X (2009) Study on fluorescence spectra of molecular association of acetic acid-water. Chin Opt Lett 7(4):357–360
Pang Y, Huang YK, Li F, Yangc FQ, **a ZN (2016) Rapid screening and evaluation of antioxidants in alkaloid natural products by capillary electrophoresis with chemiluminescence detection. Anal Methods 8:6545–6553. https://doi.org/10.1039/C6AY01518B
Farr EP, Quintana JC, Reynoso V, Ruberry JD, Shin WR, Swartz KR (2018) Introduction to time-resolved spectroscopy: nanosecond transient absorption and time-resolved fluorescence of eosin B. J Chem Educ 95(5):864–871. https://doi.org/10.1021/acs.jchemed.7b00941
Birch DJS, Imhof RE (2002). Time-Domain Fluorescence Spectroscopy Using Time-Correlated Single-Photon Counting. In: J.R. Lakowicz, (eds) Topics in Fluorescence Spectroscopy. Topics in Fluorescence Spectroscopy, vol 1. Springer, Boston, MA. https://doi.org/10.1007/0-306-47057-8_1
Grinvald A, Steinberg IZ (1974) On the analysis of fluorescence decay kinetics by the method of least-squares. Anal Biochem 59:583–598. https://doi.org/10.1016/0003-2697(74)90312-1
Acknowledgements
Monalisha Nayak would like to acknowledge UGC New Delhi for providing non net fellowship. Prof Ranjan K Singh is thankful to DST- FIST programme, and ongoing BHU IOE scheme for financial support. Authors are thankful to Prof Amit Pathak for providing computational facility.
Funding
Not available.
Author information
Authors and Affiliations
Contributions
Monalisha Nayak: Designing and execution of ideas for sample preparation, characterization, theoretical calculation as well as manuscript writing, Chandan Bhai Patel: Sample preparation, discussion of the results, review of the manuscript, Anurag Mishra: Experimental suggestion, Ranjana Singh: Ideas, experiments, results and discussion, manuscript writing. Ranjan K. Singh: Supervision for the experiment, result and discussion, manuscript writing, reviewing and editing.
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not Applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nayak, M., Patel, C.B., Mishra, A. et al. Unveiling the Influence of Glutathione in Suppressing the Conversion of Aspirin to Salicylic Acid: A Fluorescence and DFT Study. J Fluoresc 34, 1441–1451 (2024). https://doi.org/10.1007/s10895-024-03665-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-024-03665-1