Abstract
Ag mixed Cd–Cu nanoferrite (Cd0.5Cu0.25Ag0.25Fe2O4; Ag-CCF) has been successfully prepared by co-precipitation method. The grain size of Ag mixed Cd–Cu ferrite was 10 nm estimated by HRTEM; while the specific surface area was measured by BET was 161 m2/g. The XRD measurement shows a good crystallization ferrite phase and FT-IR spectrum represents two characterized bands of spinel ferrite 428 cm−1 and 596 cm−1. The three broad peaks of Raman spectrum 276 cm−1, 386 cm−1 and 643 cm−1 are characterized of spinel phase. The VSM measurement has represented superparamagnetism with saturation magnetization 30 emu/g, remnant magnetization 1 emu/g and coercive field 38G. The Ag-CCF/rGO (reduced grapheme oxide) composite used for electrode in supercapacitor deliver specific capacitance of 306 F/g at scan rate 5 mV/s. Nyquist plot represented that charge transfer resistance of the Cd–CuFe2O4/rGO composites (82.3 Ω) was much smaller than that of the pristine Cd-CuFe2O4 (591 Ω). This can be ascribed to the introduction of conductive graphene improved the electrical conductivity of the composites, leading to the better rate capability and higher specific capacitance in comparison with the pristine Cd–CuFe2O4.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Due to rapid growth of energy utilization for various developments indicates the shortage of conventional fossil fuels in near future demands to find renewable and sustainable energy to substitute traditional fossil fuels. On this aspect, considerable efforts have been made to obtain high performance containing electrochemical energy storage devices [1, 2]. As a result; electrochemical energy storage devices related research has been widely investigated to achieve high specific capacity, quick charging rate, higher energy and power density, and long life cycling stability in electronic equipment and energy fields [3]. Batteries and supercapacitors (SCs) are usually used as electrochemical energy storage application. In batteries and SCs, electrode materials having high specific surface area, superior stability and high porosity, play crucial role to improve the performance of batteries and SCs [4, 5].
Electrodes play a vital role in the performances of SCs [6]. The electrochemical stability and designing approach for the future advancement of the electrode materials are gained an impressive concentration by the researchers to improve the energy and power densities, long cyclic life, rapid charge–discharge rates, low maintenance and desirable safety [7, 8]. Hybrid electrode material CoSe2 nanorod-CuSe polyhedron-decorated graphene oxide (GO) was designed and studied for its application in high-performance hybrid asymmetric SCs. Owing to their specific architecture, high mesoporosity, and rapid transportation of electrons/ions, an asymmetric SC device with a CCS@GO//activated-carbon (AC) electrode exhibited excellent electrochemical properties that endowed it with a high specific capacitance and extensive energy and power densities [9]. Furthermore, the long-term durability over 10,000 cycles with capacitance retention of 82.5% suggests that the Hybrid electrode material has immense potential for future-generation electronic devices and hybrid electric vehicles [10]. Another metal organic–inorganic hybrid material has gained considerable attention in the fields of electrochemical energy storage and conversion devices owing to their open topological structures, large surface areas, tunable morphologies, and extreme redox activity. Polyhedron of nickel–cobalt alloy on graphitic carbon (GC; NiCo@GC) nanostructures exhibits mesoporous characteristics reveals the battery-type charge storage behavior. Owing to the reasonable electrochemical characteristics of the prepared NiCo@GC material, it is a promising candidate for supercapattery electrodes for portable electronic devices [11].
Spinel ferrites MFe2O4 (where M refers to divalent metal ions) are versatile and technologically important magnetic materials due to their excellent magnetic, catalytic and electrical properties. Especially, their different redox states and electrochemical stability can achieve high-performance energy storage. Some spinel ferrites have been used as electrodes for pseudocapacitor due to their fast and reversible faradaic redox reactions involving contributions from both the divalent metal and Fe ions. However, the low electrical conductivity significantly affects their charge–discharge performance. Recently, it was demonstrated that the combination of spinel ferrite and graphene is beneficial to improve the electrochemical performance of materials.
Transition metal oxides with carbon nanostructures are promising for energy storage devices because of their pseudocapacitance behavior with greater stability [12,13,14]. In pervious study, Cu2O and CdFe2O4 nanoparticles (CdFe nanoparticles) were placed on g-C3N4 sheets using a complicated process. The synthesized hybrid composites were assessed as SCs with symmetric and asymmetric setups. The electrochemical performance of Cu2O@CdFe nanoparticles was much higher than the Cu2O, CdFe nanoparticles. The superior composition and their remarkable electrochemical performance suggest that the synthesized composite is favorable for SC applications [15]. Asymmetric SC was effectively fabricated using Ag@rGO nanocomposite as positive electrode and the Graphene Oxide as negative electrode with polyvinyl alcohol (PVA)/6 M KOH electrolyte. The asymmetric SC assembly of Ag@rGO//rGO hybrid energy storage device provided a wide voltage window and showed good cycle stability. The capacitance retention finished at 500 cycles [16].
Ag/MnO2/PANI (silver/manganese oxide/polyaniline) nanocomposites were synthesized for SC applications. The electrochemical SC properties of the Ag/MnO2/PANI nanocomposite obtained from the cyclic voltammogram (CV) is much higher than that of the pure PANI [17]. In this regard, an attempt was made to synthesis non-stoichiometric Ag mixed Cd–Cu ferrite nanoparticles for achieving maximum capacitance with extensive operating voltage and high rate performance in SCs. We adopt a simple and scalable chemical co-precipitation method to obtain the nanoparticles. The resultant Ag0.25Cd0.5Cu0.25Fe2O4 nanosize is characterized for their structure, morphology and their electrochemical applications. The Ag+1 mixed Cd–Cu ferrite and Ag0.25Cd0.5Cu0.25Fe2O4/rGO nanocomposite were synthesized. The electrochemical performances of as-obtained samples were investigated and compared for the first time. Asymmetric SC was effectively fabricated using Ag@rGO nanocomposite as positive electrode and the Graphene Oxide as negative electrode with polyvinyl alcohol (PVA)/6 M KOH electrolyte. The asymmetric SC assembly of Ag@rGO//rGO hybrid energy storage device provided a wide voltage window and showed good cycle stability. The capacitance retention finished at 500 cycles [16].
Recently, preparation of electrode materials for SCs by integration of multi-metal/metal oxide components has drawn widespread attention due to their specific physical/chemical properties, such as electronic conductivity, crystal structure, electrochemical activity etc. [18,19,20,21]. Multi-metal/ metal oxide with hierarchical crystal structures exhibited superior electrode performance to those of their individual building blocks. Among the numerous metal oxides, spinel-type transition metal ferrites (MFe2O4, M = Fe, Co, Ni, Mn, Zn) have received a lot of attention as promising electrode materials because of their environmental safe, good electrical properties, thermal stability, and chemical manipulation ability with a wide range of applications [22,23,24,25,26,27,34], Co ferrite [35], magnetite [36], Mn ferrite [37], Ni–Co ferrite [38] and Ag complexes [39, 40]. However, the low surface area and energy density resulting from the electrical conductivity of these materials; the use of a mixture of transition metal oxides with reduced graphene oxide in hybrid SCs improved the electrical properties [41,42,43,44,45,46].
Among the various metal ferrites, Cd–Cu ferrite magnetic nanoparticles (Cd0.5Cu0.25Ag0.25Fe2O4) were synthesized by co-precipitation methods using precursors as nitrates. In various spinel ferrites, the Cd–Cu ferrite (Cd0.5Cu0.5Fe2O4) has been widely studied exhibiting good magnetic and dielectric properties. In the current work, electrode materials for SC has been synthesized by Ag mixed Cd–Cu ferrite by combining high specific surface area containing reduced graphene oxide (rGO). The composites have been synthesized via a simple physical mixing and characterized using XRD, FT-IR, Raman, HRTEM, and FESEM, and their supercapacitance measurement have evaluated using potentiostat and galvanostat.
2 Experimental details
2.1 Synthesis of Ag mixed Cd–Cu nanoferrite mixed reduced graphene oxide
Cd0.5Cu0.5−xAgxFe2O4 (x = 0.0, 0.1, 0.2, 0.25, 0.3, 0.4 and 0.5) nanoferrite samples were synthesized by wet-chemical co-precipitation method. The starting solutions were prepared as 0.2 M of cadmium nitrate Cd (NO3)2.4H2O, copper nitrates Cu (NO3)2.4H2O; and silver nitrate Ag (NO3) and 0.4 M of ferric nitrate Fe (NO3)3.9H2O. These solutions were mixed at 50 °C then 3 M of sodium hydroxide NaOH was added to solution drop wise. After that, the mixture was stirred for about 2 h at 80 °C and then cooled to room temperature. The precipitate was obtained from a magnetic separation process, and the final product was washed with water before being dried in drying furnace at 80 °C for 12 h. The Ag–CCF/rGO composite materials were prepared by mixing 1 mg of rGO nanosheets with 1 mg Ag mixed Cd–CuFe2O4 sample in 2 ml of the prepared solvent.
2.2 Characterization
The structural and morphological analyses of synthesized materials were extensively analyzed using different techniques. Crystallographic structural analysis of samples were explored using X-ray powder diffraction (XRD) on Rigaku RINT-2500 X-ray diffractometer using Cu Kα (1.54 Å) with an acceleration voltage of 40 kV and current of 20 mA. Fourier-transform infrared (FT-IR) spectra have been recorded in the wave number range of 200–1000 cm−1 by a spectrometer of the type Bruker Tensor 27. Field-emission scanning electron microscopy (FE-SEM, Hitachi SU 8000 Type II) combined with energy dispersive X-ray spectroscopy (EDS) as well as transmission electron microscopy (TEM, JEM-2100 F, JEOL) have been used to assess the surface morphological nanostructures of the synthesized materials. Raman spectra were acquired using a JASCO NRS-1000 micro-spectrometer using an Ar+ green laser and also an excitation wavelength of 514.5 nm. Brunauer–Emmett–Teller measurement (BET) was used (Micromeritics, USA) to quantify the surface area at 77 K.
2.3 Electrochemical measurements
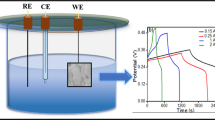
Electrochemical measurements of the as-synthesized samples were carried out by three-electrode cell system using a Solartron SI 1286 electrochemical workstation in a 1 M KOH electrolytic basic solution. In order to prepare the working electrodes, 2.0 mg of Ag-CCF/rGO composite was diffused in 2.0 ml of a 5:5:0.1 volume ratio slurry of isopropyl alcohol, water, and nafion with the assistance of ultrasoncation (15 min). With the support of a micro-syringe, 25 µl (2.5 µg) of the dispersed solution was then deposited onto a pristine glassy carbon electrode (GCE) with a 3.0 mm in diameter, and the solvent was then evaporated in the air to dry the active materials. In three-electrode tests, platinum (Pt) wire has been used as a counter electrode, Ag–CCF/rGO composites as the working electrode and Ag/AgCl was the reference electrode. The electrochemical performance of the Ag–CCF/rGO composite electrode for SCs applications was investigated utilizing cyclic voltammetry (CV), galvanostatic charging and discharging (GCD), and electrochemical impedance spectroscopy (EIS) measurements. The CV data were measured at several scan rates ranging from 5 to 100 mV/s in the potential window of − 0.2 to 0.8 V. The electrochemical impedance spectroscopy (EIS) measurements were made with an open circuit potential in frequency range of 100 kHz to 10 mHz.
3 Results and discussion
3.1 Structural and morphological characterization
XRD is an efficient tool that can give important information about structural properties of materials. Figure 1a displays the crystallographic indices (220), (311), (400), (422), (511), (440), and (533) with diffraction angles between 20° and 80°. XRD patterns for Cd0.5Cu0.25Ag0.25Fe2O4 corresponding to the characteristic peaks of the cubic spinel crystalline structure Cd0.5Cu0.5Fe2O4 (JCPDS No. a: 22-1086, b: 10-0325, c: 10-0319, d: 22-1012) [47]. The other diffraction peaks indicate the presence of the silver complexes, correlating with previous results reported in the literature. Therefore, the XRD charts of the samples contain characteristic peaks of nanocomposites that can be indexed as the majority phase of cubic spinel nanoferrite phase (JCPDS No. a: 22-1086, b: 10-0325, c: 10-0319, and d: 22-1012) and a minority phase of Ag complexes [40]. Clearly, the competition between the two phases appears through the broadening of the diffraction peak at 35°. This broad peak can be fitted into two mean peaks: the cubic spinel phase (311) and the common R/H delafossite phase (102). It is important to note that the diffraction patterns exhibit a lattice parameter (0.8479 nm) in the order of the Cd0.5Cu0.5Fe2O4 compound (0.8475n m) [47]. This lower unexpected value may be attributed to the formation of Ag complexes within the spinel network’s boundaries, revealing non-stoichiometry in the formed spinel phases.
The FT-IR spectra of ferrite were used to examine nature of bonds and their modes of vibration. The FT-IR spectra of ferrite sample in the wave number range of 200 to 1000 cm−1 is shown in Fig. 1b. According to Waldron, all spinel ferrites exhibit the two strong absorption bands that could be seen at 596 cm−1, which correlate to the oscillation of the tetrahedral metal oxygen bond, and 428 cm−1, which correspond to the octahedral metal oxygen bond. The presence of these two absorption bands in spectrum confirmed the formation of spinel structure of ferrite. The spectrum also shows absorption band around 861 cm−1 is attributed to some Ag+ in the tetrahedral sites [48], while the band around 235.5 cm−1 belongs to the lattice vibrations of the system; it depends on the mass of the A-site divalent ions and their complexes [48].
The phase purity of the synthesized Ag substituted ferrite material has been investigated using Raman spectra measurement. The possibility of formation of Ag complexes is difficult to rule out. Since the spinel ferrite exhibits five Raman active modes, which become representative of the Fd3m space group, they could be seen experimentally in the 100 to 800 cm−1 wavenumber range (Fig. 1c). In conformity with the broad peaks of XRD, the Ag0.25Cu0.25Cd0.5Fe2O4 sample in Raman spectra demonstrated significant peaks with large shoulders pointing further toward the sample fine grains. Three powerful broad bands are detected in the spectrum at 276, 386, and 509 cm−1, and other three relatively moderate but intense peaks at 564 cm−1, 604 cm−1, and 643 cm−1 [49]. Higher spectral bands appear at 604 cm−1 and 643 cm−1 can be traced to Ag modes that originate from oxygen symmetric stretching and metal ions from tetrahedral vacancies that replicate the local lattice effect in the lattice sites [49]. Asymmetric and symmetric bending of the oxygen and metal ions situated in the octahedral sites corresponding to T2g(1), Eg, T2g(2) and T2g(3), respectively, is just what is accountable for such peaks at lower wavenumbers of 276 cm− 1, 386 cm− 1, 509 cm− 1, and 564 cm− 1 [50].
Figure 1d shows the magnetic hysteresis loops of Ag0.25Cu0.25Cd0.5Fe2O4 cubic spinel carried out at room temperature with a peak field of 8k Oe. As it can be seen, the variation of magnetization as a function of applied field presented small remnant magnetization (0.05 emu/g) and small coercive field (30 G) revealing a characteristic behavior of superparamagnetic nanomaterial. The saturation magnetization of the sample displayed unsaturated behavior in the measured range. The low value of magnetic saturation of Ag mixed samples (30emu/g) compared to Cu0.5Cd0.5Fe2O4 (45 emu/g) can be attributed to the Ag do** in the spinel structure [51]. This result is in good agreement with previous reports for Ag complexes with the spinel ferrite in nanocomposites [52, 53]. Interestingly, the material activity increase compensates the significant magnetic decrease of silver do** nanospinel ferrite.
Surface morphology and the shape of the synthesized nanoparticles were identified by SEM and TEM characterization. From Fig. 2a–d, the prepared ferrite samples are composed of agglomerated particle with different particle sizes deposited to different extent. The grains are not well crystallized which give an agreement with the broadening of some peaks in XRD patterns [54]. The particles agglomerated in cluster form and the aggregation of the particles indicating strong interaction between magnetic nanoparticles, weak Vander Waal’s interaction and high surface energy in powder form [55].
EDX spectra provides information about the elemental composition present in materials from the surface to the internal of the solids and it is used to affirm the homogeneity of the sample [51]. Figure 2c represents a typical EDX of Cd0.5Cu0.25 Ag0.25Fe2O4 sample. From the Fig. 2c; it’s evident that there is agreement between the results obtained from EDXs analysis and the amount of different elements in the ferrite sample. The compositional analysis data for the samples reveal the presence of Cu, Cd, Ag, Fe, O elements in the spinel structure of sample without any impurities which proved the completely removal of the undesirable materials.
Do** of monovalent ion Ag1+ instead of the divalent ion copper Cu2+ produced spherical shape of non-stoichiometric silver ferrite sample in agglomerated homogeneous structure. This structure appears clearly by HRTEM in Fig. 2b. From the selected area electron diffraction pattern (SAED) in Fig. 2d enclosed groups of circles are attributed to the crystalline cubic nanoferrite structure. Additionally; this result did not conflict with the formation of nanoclusters Ag complexes in the limits of non-stoichiometric spinel network supporting the XRD pattern broadening around 2θ = 35° representing the amorphous phase for the sample.
Using SEM, the morphological properties of rGO nanosheets and Ag–CCF/rGO composite were examined. The SEM images of the rGO nanosheets, that revealed layered structures with considerable surface area and low thickness, could be seen in Fig. 3a. The prepared specimen includes open boundaries and porous rGO nanosheets that seem to be evident in the SEM image (Fig. 3a). A three-dimensional (3D) interlinked porous nanostructure of rGO nanosheets is located at the accessible boundaries. High resolution TEM image in Fig. 3b shows highly transparent and wrinkled rGO nanosheets implying few number of graphene layers. High resolution TEM image reveals a slender nanostructure with an interlayer spacing of 0.34 nm and blurriness of graphene, which explicitly states that rGO nanosheets only contain less number of layers of graphene. SEM image of Ag–CCF/rGO composites have been shown in Fig. 3c, wherein ferrite nanoparticles are attached on rGO nanosheets. High resolution TEM studies were performed to provide further details on the interior nanostructure and crystallinity of the Ag–CCF/rGO composites. According to Fig. 3d, ferrite nanoparticles were connected to the rGO nanosheets surface, which has thin transparent wrinkles. It demonstrates that the thin rGO nanosheets were covered by Cd0.5Cu0.25Ag0.25Fe2O4 nanopaticles and the size of nanoparticles was approximately 100 nm as can be seen in Fig. 3d.
Nitrogen adsorption–desorption method utilizing BET measurement has been used to evaluate the specific surface area and pore diameter of synthesized materials as rGO nanosheets and Cd0.5Cu0.25Ag0.25Fe2O4 nanoparticles. This measurement confirms that the prepared rGO nanosheets has high surface area (570 m2/g) (Fig. 4a). The specific surface area of Ag ferrite was 161 m2/g, as can be seen in Fig. 4b. In this regard, even though the specific surface area of rGO/Ag ferrite hybrids is smaller than that of rGO nanosheets due to formation of Ag ferrite nanoparticles into open margins on rGO nanosheets.
3.2 Electrochemical studies
In order to examine the electrochemical performance of rGO nanosheets and Ag–CCF/rGO composite as active material in SC, Fig. 5 contains the CV curves. As can be seen in Fig. 5a, b, the CV curves for Ag ferrites and Ag–CCF/rGO composite were measured at different scan rates (from 20 to 100 mV/s) within in the potential window from 0.2 to + 0.8 V. At the same scan rates, the Ag–CCF/rGO composite electrode CV curve (Fig. 5b) seemed to have a larger area and nearly rectangular CV curve (Fig. 5a) as compared to Ag ferrite. It illustrates that the area increased and the specific capacitance improved following the addition of rGO nanosheets to Ag ferrite. The current increases as the scan rate increases as well as a sudden variation in the redox reactions peaks emerges. The above suggests that the synthetic composite exhibit strong electrochemical reversibility and expanded potential separation [56]. The Ag–CCF/rGO composite CV curve form exposes the less contribution of pseudocapacitance characteristic with high contribution from EDLCs.
Specific capacitances were calculated from Fig. 5b at different scan rate as estimated by the CV curve. At the respective scanning speeds of 5, 10, 20, 30, 40, 50, 60, 70, 80, 90, and 100 mV/s, the specific capacitances of Ag–CCF/rGO composite electrodes comprised 306, 240, 214, 195, 181, 165, 164, 154, 151, 149, and 143 F/g. In contrary, as the scanning rates increases from 5 mV/s to 100 mV/s, the specific capacitance of the Ag-CCF/rGO composite electrode decreases from 306 F/g to 143 F/g. In addition, compared to the scanning speed of 100 mV/s, the specific capacitance of the composite electrode was risen steadily 2.14 times. Owing to the inclusion of Ag ferrites in the porous layers of the rGO nanosheets, it validated the remarkable rate performance of the Ag–CCF/rGO composite electrode.
Galvanostatic charging and discharging (GCD) of Ag–CCF/rGO composite electrode at different current densities has been enabled for further investigation. Figure 6a depicts the GCD profile of Ag–CCF/rGO composite electrode at various current densities of 0.1, 0.15, 0.2, and 0.3 A/g. The GCD curves gradually shrunk as current density increases from 0.1 to 0.3 A/g. The electrode has presented nonlinear GCD curve characteristics at various current densities support the hypothesis that the redox reactions were consistent with CV curves. The pseudocapacitance generated by the redox reactions of Ag ferrites situated between the layers of rGO nanosheets coincides to the plateau’s occurrence in the GCD curve.
Figure 6c, shows the dependence of area on scan rate obtained from using CV curve (Fig. 5b). The area increases with scan rate but in comparison, the specific capacitance of Ag–CCF/rGO composite electrode decreased with increase of scan rates from 5 mV/s to 100 mV/s. the increase in area is not straight proportional with the scan rate.
Using GCD measurements, it is possible to test the stability, another crucial component that has an impact on the actual application of SCs (Fig. 6c). Ongoing about 2000 measures have been used to verify the stability of the Ag–CCF/rGO composite electrode (current density: 0.3 A/g). The Ag–CCF/rGO composite electrode preserved very stable charging discharging measure, proving that the composite electrode has quick stability for SCs application.
Using CV measurements, also it is possible to test the stability, another crucial component that has an impact on the actual application of SCs. Ongoing 2000 cycles have been used to verify the cycle stability of the Ag–CCF/rGO composite electrode (scan rate: 100 mV/s). The Ag–CCF/rGO composite electrode preserved 88.9% of own capacitance after 110 cycles, proving that the composite electrode has quick stability for SCs application. After 2000 cycles, the rGO/Ag composite electrode’s specific capacitance declined from 143 to 116 F/g with retention of 81.1%. Implying the possibilities of Ag–CCF/rGO composite electrodes as a highly efficient SCs electrode material for extremely stable for long cycles, about 81.1% of the initial capacitance remaining persisted. The broad porous structure surface containing rGO NSs, which offers the large surface areas and enhancement in ion mobility via CV test for repeated cycles, may be the reason for the increased retention as well as long cycling stability.
The electrochemical characteristics were investigated employing electrochemical impedance spectroscopy (EIS) analysis. The most essential graph in the EIS analysis is the Nyquist plot of the materials utilised for SCs electrode. The pattern reveals the electrode-electrolyte system’s operating frequency for the real (Z′) and imaginary (− Z′′) parts of the impedance. The Nyquist plot in the frequency range of 0.1 Hz to 300 kHz with an AC voltage magnitude of 10 mV can be seen in Fig. 6d. The diameter of the semicircle is a direct indication of charge transfer resistance (Rct), which would be the interface resistance of the electrode materials/electrolyte, and the intercept of the Z′ axis in the high-frequency range coincides to solution resistance (Rs) (ionic resistance from electrolyte, intrinsic resistance from substrate, and contact resistance on the interface of active material/current collector) [57, 58]. As can be noticed, hybrid rGO/Ag mixed electrode has low values of Rs (87.4 Ω) and Rct (8.5 Ω), signifying a short electron path and close contact between the highly conductive rGO nanosheets and Ag nanoparticles. The diffusion of ions in the electrode materials can be connected to the steep straight line at low frequencies.
Despite the EIS of both electrodes behaving similarly, there was a noticeable difference in the equivalent circuit at low frequencies due to the discrepancy in the values. The best fit of the pristine electrode was determined to have a capacitor, C, while that of the composite electrode contained Warburg resistance. According to this fitting, Ag–CCF/rGO has a lower solution resistance (Rs 37.3) than pristine Ag–CCF (Rs 84.47), which aids in the faster transaction of their ions. The charge transfer resistance is dealt by the obtained semicircle region, and the order of charge transfer resistance varies as rGO cooperate in the electrode, indicating that Ag–CCF/rGO exhibited better ion diffusion than pristine Ag–CCF. The lower charge transfer resistance of Ag–CCF/rGO (Rct 82.33) compared to pristine Ag–CCF (Rct 591.29) revealed improved ion diffusion at the electrode-electrolyte interface. At the low frequency region, the capacitor (C) present in conjunction with the pristine Ag–CCF electrode may be attributed to the presence of electrode polarization, which is clearly audible at low frequencies and vanishes at high frequencies. At the high-frequency region, the Warburg resistance of the Ag–CCF/rGO composite reveals the catalytic property of graphene oxide. This catalytic property is the main reason for increasing the amount and rate of charges moving in the electrolyte, which increases the conductivity and capacitance of the composite electrode. In comparing to previously publications, the achieved electrochemical performance of the Ag–CCF/rGO composite electrode (Table 1) exhibits considerably improved electrochemical performance (specific capacitance and long cycling stability).
4 Conclusion
Cubic spinel nanoparticle Cd−Cu ferrite mixed Ag (Cd0.5Cu0.25Ag0.25Fe2O4) has been successfully produced utilizing the co-precipitation method. The sample has undergone all the necessary characterizations that show it is suitable for use in SCs. By mixing high specific surface area rGO with Ag mixed Cd−Cu ferrite, we aim to improve its SC capabilities. The composites were created using a straightforward physical mixing process, and the prepared samples were characterized using XRD, FT-IR, Raman, HRTEM, and FESEM. Potentiostate/galvanostate were also used to assess the supercapacitance of the composites. The specific capacitance of the Ag−CCF/rGO composite electrode decreased from 306 to 143 F/g as the scanning rates increases from 5 to 100 mV/s.
Data availability
All data generated or analyzed during this study are included in this published article.
References
R. Kumar, S. Sahoo, E. Joanni, R.K. Singh, W.K. Tan, K.K. Kar, A. Matsuda, Prog. Energy Combust. Sci 75, 100786 (2019)
R. Kumar, E. Joanni, R.K. Singh, D.P. Singh, S.A. Moshkalev, Prog. Energy Combust. Sci 67, 115 (2018)
J. Sun, C. Liu, X. Song, J. Zhang, Y. Liu, L. Liang, R. Jiang, C. Yuan, Appl. Phys. Reviews 9, 031301 (2022)
S. Rajagopal, R. Pulapparambil Vallikkattil, M. Mohamed Ibrahim, D.G. Velev, Condens. Matter 7, 6 (2022)
R. Kumar, S. Sahoo, E. Joanni, R.K. Singh, K.K. Kar, ACS Appl. Mater. Interfaces 14, 20306 (2022)
R. Kumar, S.M. Youssry, E. Joanni, S. Sahoo, G. Kawamura, A. Matsuda, J. Energy Storage 56, 105896 (2022)
J. Theerthagiri, G. Durai, K. Karuppasamy, P. Arunachalam, V. Elakkiya, P. Kuppusami, T. Maiyalagan, H.-S. Kim, J. Ind. Eng. Chem 67, 12 (2018)
R. Kumar, A. Pérez del Pino, S. Sahoo, R.K. Singh, W.K. Tan, K.K. Kar, A. Matsuda, E. Joanni, Prog. Energy Combust. Sci 91, 100981 (2022)
R. Kumar, E. Joanni, S. Sahoo, J.-J. Shim, W.K. Tan, A. Matsuda, R.K. Singh, Carbon 193, 298 (2022)
K. Karuppasamy, D. Vikraman, S. Hussain, G. Kumar Veerasubramani, P. Santhoshkumar, S.-H. Lee, R. Bose, A. Kathalingam, H.-S. Kim, Chem. Eng. J 427, 131535 (2022)
K. Karuppasamy, D. Vikraman, S. Hussain, P. Santhoshkumar, R. Bose, P. Sivakumar, A. Alfantazi, J. Jung, H.-S. Kim, Small 18, 2107284 (2022)
R. Kumar, S.M. Youssry, H.M. Soe, M.M. Abdel-Galeil, G. Kawamura, A. Matsuda, J. Energy Storage 30, 101539 (2020)
R. Kumar, S. Sahoo, W.K. Tan, G. Kawamura, A. Matsuda, K.K. Kar, J. Energy Storage 40, 102724 (2021)
S.M. Youssry, I.S. El-Hallag, R. Kumar, G. Kawamura, W.K. Tan, A. Matsuda, M.N. El-Nahass, J. Energy Storage 50, 104638 (2022)
N.R. Reddy, P.M. Reddy, T.K. Mandal, A.K. Yedluri, S.W. Joo, J. Energy Storage 43, 103302 (2021)
W. Tuichai, A. Karaphun, C. Ruttanapun, J. Alloys Compd 849, 156516 (2020)
J. Kim, H. Ju, A.I. Inamdar, Y. Jo, J. Han, H. Kim, H. Im, Energy 70, 473 (2014)
H. Chen, T.N. Cong, W. Yang, C. Tan, Y. Li, Y. Ding, Prog. Nat. Sci 19, 291 (2009)
R. Kumar, R.K. Singh, D.P. Singh, E. Joanni, R.M. Yadav, S.A. Moshkalev, Coord. Chem. Rev 342, 34 (2017)
E.E. Miller, Y. Hua, F.H. Tezel, J. Energy Storage 20, 30 (2018)
W. Raza, F. Ali, N. Raza, Y. Luo, K.-H. Kim, J. Yang, S. Kumar, A. Mehmood, E.E. Kwon, Nano Energy 52, 441 (2018)
P. Anggo Krisbiantoro, Y.-W. Chiao, W. Liao, J.-P. Sun, D. Tsutsumi, H. Yamamoto, Y. Kamiya, K.C.-W. Wu, Chem. Eng. J 450, 137926 (2022)
H. Belhadj, Y. Messaoudi, M.R. Khelladi, A. Azizi, Int. J. Hydrog. Energy 47, 20129 (2022)
M. Kazemi, Synth. Commun 50, 1899 (2020)
L. Lv, Y. Wang, P. Cheng, Y. Zhang, Y. Zhang, Z. Lei, L. Xu, Z. Weng, J. Alloys Compd 904, 164054 (2022)
S. Qiao, N. Zhang, H. Wu, M. Hanas, Synth. Commun 51, 2873 (2021)
M. Saha, S. Mukherjee, P. Bera, M.M. Seikh, A. Gayen, Ceram. Int 48, 35719 (2022)
X. **e, B. Wang, Y. Wang, C. Ni, X. Sun, W. Du, Chem. Eng. J 428, 131160 (2022)
S. Zhang, J. Wu, F. Li, L. Li, J. Environ. Chem. Eng. 10, 107702 (2022)
R. Kumar, R.K. Singh, A.K. Singh, A.R. Vaz, C.S. Rout, S.A. Moshkalev, Appl. Surf. Sci 416, 259 (2017)
R. Kumar, M.M. Abdel-Galeil, K.Z. Ya, K. Fujita, W.K. Tan, A. Matsuda, Appl. Surf. Sci 481, 296 (2019)
S. K, D.B.D. Madhavaprasad, J. Indian Chem. Soc 99, 100565 (2022)
M. Kurian, S. Thankachan, Open Ceram. 8, 100179 (2021)
R. Tamilselvi, G.S. Lekshmi, N. Padmanathan, V. Selvaraj, O. Bazaka, I. Levchenko, K. Bazaka, M. Mandhakini, Renew. Energy 181, 1386 (2022)
W.E. Mahmoud, A.A. Al-Ghamdi, Y.A. Al-Turki, A.M. Al-Amri, A. Alshahrie, J. Alloys Compd 900, 163564 (2022)
J.R. Rajabathar, J.J. Vijaya, A. Prabakaran, Z.A. Issa, A.M. Atta, A.O. Ezzat, A.M. Al-Mayouf, H.A. Al-Lohedan, J. Alloys Compd 698, 1077 (2017)
W. Cai, T. Lai, W. Dai, J. Ye, J. Power Sources 255, 170 (2014)
J.-L. Ortiz-Quiñonez, U. Pal, M.S. Villanueva, ACS Omega 3, 14986 (2018)
J.L. Durham, C.J. Pelliccione, W. Zhang, A.S. Poyraz, Z. Lin, X. Tong, F. Wang, E.S. Takeuchi, A.C. Marschilok, K.J. Takeuchi, Appl. Mater. Today 10, 142 (2018)
I.M. Babu, K.K. Purushothaman, G. Muralidharan, J. Mater. Chem. A 3, 420 (2015)
M.A. Deyab, A.E. Awadallah, H.A. Ahmed, Q. Mohsen, J. Energy Storage 46, 103926 (2022)
K. Hareesh, S.R. Rondiya, N.Y. Dzade, S.D. Dhole, J. Williams, S. Sergey, J. Science: Adv. Mater. Devices 6, 291 (2021)
S. Martinez-Vargas, A.I. Mtz-Enriquez, H. Flores-Zuñiga, A. Encinas, J. Oliva, Synth. Met 264, 116384 (2020)
B. Ding, X. Wu, J. Alloys Compd 842, 155838 (2020)
W.K. Tan, K. Asami, K. Maegawa, R. Kumar, G. Kawamura, H. Muto, A. Matsuda, Mater. Today Commun. 25, 101540 (2020)
W.H. Low, P.S. Khiew, S.S. Lim, C.W. Siong, E.R. Ezeigwe, J. Alloys Compd 775, 1324 (2019)
R. Rai, K. Verma, S. Sharma, S.S. Nair, M. Almeida Valente, A.L. Kholkin, N.A. Sobolev, J. Phys. Chem. Solids 72, 862 (2011)
A. Ananth, Y.S. Mok, Nanomaterials 6, 42 (2016)
P. Chandramohan, M.P. Srinivasan, S. Velmurugan, S.V. Narasimhan, J. Solid State Chem 184, 89 (2011)
P.S.J. Bharadwaj, K.P. Gannavarapu, V.S. Kollipara, R.B. Dandamudi, J. Energy Storage 36, 102444 (2021)
R. Rai, K. Verma, S. Sharma, S.S. Nair, M.A. Valente, A.L. Kholkin, N.A. Sobolev, J. Alloys Compd 509, 7585 (2011)
B. Aslibeiki, Curr. Appl. Phys 14, 1659 (2014)
D.-H. Zhang, H.-B. Li, G.-D. Li, J.-S. Chen, Dalton Trans. 10527 (2009). https://doi.org/10.1039/B915232F
S. Thankachan, M.V. Femsy, N. John, Mater. Today Proc. 25, 289 (2020)
K. Tanbir, L.K. Sharma, R.K. Aakash, R.K. Singh, S. Choubey, Mukherjee, J. Magn. Magn. Mater 456, 118 (2018)
R. Kumar, R.K. Singh, P. Kumar Dubey, D.P. Singh, R.M. Yadav, R.S. Tiwari, RSC Adv 5, 7112 (2015)
R. Kumar, E. Joanni, R.K. Singh, E.T.S.G. da Silva, R. Savu, L.T. Kubota, S.A. Moshkalev, J. Colloid Interface Sci 507, 271 (2017)
Y. Sun, W. Zhang, D. Li, L. Gao, C. Hou, Y. Zhang, Y. Liu, J. Alloys Compd 649, 579 (2015)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This project was supported financially by the Academy of Scientific Research and Technology (ASRT), Egypt, Grant No. 6463.
Author information
Authors and Affiliations
Contributions
REES took the lead in planning and writing the manuscript on this work. RK synthesized reduced graphene oxide material. MMA-G helped in measuring the CV and GCD for the electrodes and its physical, electrochemical analysis. HSE-D supervised the experiments and data analysis methods and advised to structure this manuscript. TMM provided comprehensive support from acquiring research funding to establish the research concept, experimentation, discussion, and writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El Shater, R.E., El-Desoky, H.S., Meaz, T.M. et al. Electrochemical investigation of Ag mixed Cd–Cu nanoferrite mixed reduced graphene oxide as improved platform for supercapacitor application. J Mater Sci: Mater Electron 34, 842 (2023). https://doi.org/10.1007/s10854-023-10023-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-10023-7