Abstract
A multilayer thin film from zinc oxide and silver (ZnO/Ag multilayer thin film) has been synthesized via the technique of pulsed infrared laser deposition at 600 °C to be used as a portable catalyst for the degradation of 4-nitrophenol. The multilayer thin film was formed by two steps; the first one was making a thin film with a 300 nm thickness, followed by a 100 nm thin film from Ag, which was characterized by different characterization techniques. The XRD data demonstrated the presence of hexagonal Ag on ZnO in the formation of ZnO thin films and ZnO/Ag thin films. Also, it showed that crystallite size is decreasing as the Ag concentration rises because of the difference in atomic radius between Zn and Ag atoms in the crystal structure formation of ZnO coated with Ag. The crystal quality of ZnO and Ag was evaluated using photoluminescence (PL). The optical investigation showed the decrease in transmittance after coating ZnO film with Ag was due to ZnO films including more voids than ZnO/Ag films. From SEM images, the morphology of ZnO films was modified by the appearance of brilliant spots, which was related to the coating of the Ag layer as confirmed by elemental analysis. Then, based on the findings of catalytic experiments against 4-nitrophenol, the ZnO/Ag multilayer thin film shows an exceptional potential enhancement in compared to that of ZnO thin film.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Industrial operations are the largest and leading cause of water pollution due to the production of pollutants of various qualities. Typical water pollutants include pesticides, volatile hazard organic compounds, heavy metals, nitrates, and radionuclides. Furthermore, water may contain many disease-causing organisms, such as bacteria, viruses, and parasites, which must be eradicated or inactivated to assure the safety of those who will reuse the water [1,2,3,4,5]. The new invention is essential for facilitating sustainable water management. In addition, novel strategies and approaches for the cleaning and recycling of industrial wastewater must be supported by eliminating the use of chemicals and reducing waste generation. To meet the challenges of getting sufficient removal of contaminants without generating dangerous byproducts with conventional chemical disinfectants and to meet the rising demand for water treatment and recycling systems, new technologies for effective disinfection and microbial control are required.
Several researchers have examined the use of nanoparticles, such as silver and zinc oxide, in disinfection water treatment and microbial control applications of nanotechnology. However, the presence of nanostructured materials after water treatment may pose an additional issue that must be addressed. Nanoparticles can be immobilized on different types of organic and inorganic matrices to reduce their mobility and avoid their appearance in the environment, enabling the manufacture of antibacterial materials and highly efficient adsorption materials for use in water treatment devices [5,6,7,8]. Therefore, the combination of NPs with other nanoparticles in a composite structure for water purification is an exciting nanoparticle recovery technique. The layer-by-layer self-assembly process is an effective method for fabricating thin films and a straightforward method for immobilizing NPs of controllable size and amount of material deposited or injected into the polymeric network. Besides, one of the best aspects of these materials is that they may be reused in the water disinfection process [9,10,11,12,13].
Silver (Ag) has been considered as one of the best electrical conductors for a number of years, and it has a variety of scientific and technological applications. Due to their unique optical and antibacterial properties, silver thin films have been exploited for surface plasmon resonance (SPR), which has numerous applications in biology, medicine, and the environment. In other words, semiconducting materials such as titanium dioxide (TiO2) and zinc oxide (ZnO) have been intensively studied in water treatment as catalytic materials. ZnO, which has a comparable band gap to TiO2 (about 3.2 eV), has been employed as an attractive option material for catalytic applications due to its physical and chemical stability, high oxidative capacity, low cost, and accessibility [5,6,7,8]. In addition, it has a higher binding energy of 60 meV per excitons, enabling its use in an extensive array of research areas, including sensors, optoelectronics, dye-sensitizations, and biological applications. Besides, previous research has shown that ZnO cannot be used directly in industrial uses due to the material's lack of optical and electrical properties, which are produced by point defects like oxygen vacancies. To enhance the features of ZnO, to alter the visible-light absorption range, the optical bandgap of ZnO nanoparticles (NPs) must be controlled by active dopant compounds. Thus, the addition of Ag to ZnO materials increases the catalytic decomposition of organic hazard pollutants in air or in aqueous media [14,15,16,17,18]. These semiconductors perform better as a result of Ag atoms acting as electron sinks, capturing photo-excited electrons and boosting charge separation state yield and duration [19,20,21,22].
Several processes, including chemical vapor deposition, sol–gel, electron gun beam evaporation, radio frequency, and direct current sputtering, can be used to produce ZnO and Ag thin films. Pulsed laser deposition (PLD) has received considerable attention among these techniques as it has multiple advantages, including no agglomeration, good purity, and an adequate and adjustable coating rate. It is frequently utilized. Due to the increased energy of condensing particles, this method of deposition enables stronger adhesion of thin films to substrates, which is favorable for industrial applications [23,24,25,26].
Therefore, ZnO thin film was studied with quick catalytic applications in mind, considering the accidental or intentional poisoning of water resources for military or terrorist purposes. Moreover, and taking into account the benefits of using catalysts in fixed supports with an Ag layer, the most effective dye catalyst discovered was immobilized and contained a mixture of phenolic compounds that can serve as a model for a number of typical hazardous compounds. Also, in order to boost the efficiency of the catalysts, ZnO thin films were loaded with Ag layers using the PLD process to enhance the efficiency of the ZnO thin film against 4-nitrophenol.
2 Material and method
2.1 Materials
4-nitrophenol, sodium borohydride, zinc dust target (5 × 5 × 2 mm3), and silver target (4 × 4 × 2mm3) were supplied by Merck Company.
2.2 Preparation method
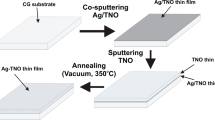
As previously indicated, the thin film was created via pulsed laser deposition of the metal target on a quartz substrate at 600 °C. The laser's employed specifications are 1064 nm wavelength, 7 ns pulse width, 30 min deposition time, 8 W average power, and 10 Hz reputation rate. From Fig. 1, PLD was initially used to generate ZnO thin films on Zn targets, then on the targets of Ag to create ZnO/Ag multilayer thin films. For Ag-coated ZnO thin film, the film thickness was determined using a profilometer (SurfTestSJ-301) and was found to be 300 nm for ZnO thin film and 100 nm for Ag.
2.3 Characterization techniques
X-ray diffraction (XRD, Schimadzu 7000, Japan), absorption spectrophotometer (JASCO 570, Japan), photoluminescence spectrometer (JASCO, FP-6500, Japan), scanning electron microscopic and energy-dispersive X-ray (SEM, FEG 250, FEI, Czech Republic) are used.
3 Result and discussions
3.1 Physicochemical study
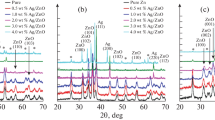
Figure 2 exhibited the diffractogram of the prepared ZnO thin film and the coating with Ag thin films on ZnO thin film to produce a multilayer thin film structure using PLD at 600 °C. In the case of the deposition of just one ZnO layer as a thin film on a quartz substrate, the number of diffraction peaks matches the reflection planes (100), (002), (101), (102), (110), and (103) associated directly with the XRD diffraction pattern of pure ZnO. (JCPDS card No. 80–0075). The deposited films have a significant orientation along the (101) -axis [27]. There are no phases in the diffractogram for ZnO thin films that correspond to any other oxides save ZnO, which proved the high purity formation of ZnO thin film. In other words, when the ZnO thin film is coated with Ag layer, three XRD peaks of the (101), (111), and (200) planes occur, which are indexed to silver that has fcc phase-matched of Ag depending on JCPDS card N. 04–0783 card. [28]. Besides, the main characteristic diffraction peaks of ZnO still appeared in the diffractogram spectrum. These observations confirmed the successful formation of the ZnO/Ag multilayer structure. In addition, the Scherrer formula was employed to calculate the crystallinity of the (101) plane, which has the largest distinctive peak [29, 30].
where λ is the wavelength of X-ray 1.54 Å, β is FWHM and θ is the angle of diffraction for the (101) plane. The crystallite size is decreasing from 40 to 21 nm as the Ag concentration rises. It demonstrates that particle size reduces as Ag concentration increases. Because of the difference in atomic radius between Zn and Ag atoms, Zn atoms are often replaced by Ag atoms in the crystal structure of ZnO coated with Ag. This means that the crystallinity of the films is dependent on the material's grain development. Besides, the PLD system performed to prepare the ZnO structure aligned around (101), which has barely been reported by different techniques, rather than (002), which was produced by other techniques.
Figure 3 illustrates SEM images of ZnO and ZnO/Ag multilayer thin films. Typically, PLD of ZnO films yields dense films. It was evident that the brilliant spots were a result of the ZnO thin film being coated with Ag film, indicating that Ag film can modify the morphology of ZnO films. Using EDX spectra, the elemental composition of ZnO/Ag multilayer thin films was investigated to confirm the presence of an Ag layer on the ZnO film. Figure 3 depicts a typical EDX measurement result for thin films. For ZnO thin film, the principal components were identified as Zn (56.67%) and O (43.33%), but for ZnO/Ag thin film, the principal components were Zn (52.41%), O (40.75%), and Ag (6.84%). The absence of any other elements in both spectra proved the great purity of both methods of film production.
In Fig. 4, the average UV–Vis transmission spectra is employed to analyze the optical characteristics of thin films grown in the case of ZnO thin film and ZnO/Ag thin film. All prepared films have excellent transmittance in the visible spectrum. They noticed that the transmittance of coated ZnO film with an Ag layer is close to 79% for 430–700 nm, which is less than the transmittance of uncoated ZnO thin film (86%). This could be owing to the fact that ZnO films include more voids than ZnO/Ag films. Also, the significant drop in UV transparency in the multilayered film may be a result of scattering from pores and other defects that are abundant in the films. As a result of electron transitions between the VB and CB, the UV transparency of each film was drastically reduced. Besides, at 368 nm, the sample displays a pronounced excitation peak with substantial absorption strength (pure ZnO). Thus, compared to pure ZnO, Ag deposition on ZnO/Ag multilayer thin films carried the absorption edge to a longer wavelength. This shift in the absorption edge could be caused by the incorporation of Ag into the ZnO matrix. A shift in the absorption spectrum suggests particles in coated ZnO may be smaller than the excitons’ Bohr radius [31,32,33].
Figure 5 depicts the PL spectra of pure ZnO and ZnO/Ag multilayer thin films, which were determined using an UV excitation laser wavelength of (λex = 325 nm) and an emission spectrum covering 350–600 nm. The PL spectra of the samples exhibit a single luminescence peak at around 417 nm. Thin ZnO films formed on quartz by PLD were seen to emanate violet fluorescence, as described. For ZnO/Ag multilayer thin film, the spectrum appeared to range between 350 and 600 nm, fitting emission peaks at 404, 424, 444, 467, 487, and 521 nm. That was related to ZnO film defects containing the well-known oxygen vacancies, Zn vacancies, interstitial Zn, interstitial oxygen, and anti-site oxygen. The interface traps between the grains of ZnO and Ag that exist at grain boundaries and produce violet light from the transition between this level and the valence or conduction band are likely responsible for the decrease in violet luminescence. Furthermore, the position and intensity of the PL emission are affected by the Ag coating. Since Ag ions have replaced Zn ions, the strength of the peak is less than for pure ZnO [34,35,36,37].
3.2 Catalytic activity
The catalytic activity of the produced metal oxide thin film of ZnO structure and its embedding with Ag atoms against hazardous chemical compounds was investigated in the oxidation–reduction catalytic activity against hazardous compounds. One of these hazardous chemical substances is 4-nitrophenol (4-NP), which is a common occurrence in industrial settings, particularly in the agricultural sector. 4-NP is an example of a hazardous organic chemical substance. In the catalytic degradation investigation, the UV–visible spectrometer was used to help in the examination of the decomposition process that occurred on 4-NP dissolved in ultra-pure water after the addition of the produced samples with NaBH4 as the reducing agent [38,39,40].
Figure 6a displayed the spectrum of 4-nitrophenol that was dissolved in distilled water, which had two characteristic peaks that occurred at 314 and 400 nm, respectively, and how it changed when NaBH4 was added as a reducing agent. 4-nitrophenolate has a single characteristic peak at 403 nm. In an aqueous solution, the structure of the 4-nitrophenolate ion takes a very long time to transform into 4-aminophenol, which represents a chemical molecule that is not dangerous. Using a time-dependent UV–visible spectrometer, Fig. 6b demonstrated the considerable variations in the reduction time of the 4-nitrophenolate ion after the addition of the produced materials. It shows that the degradation of ZnO thin films takes about 55 min for materials containing more than 90% 4-NP, while In Ag-coated ZnO multilayer thin films, the characteristic peak of 4-nitrophenolate ion starts to decrease and another peak starts to increase at about 300 nm, which represents the characteristic peak of 4-aminophenol. So, it demonstrates that the degradation process requires around 25 min to completely degrade more than 90% of 4-NP.
4 Conclusion
In conclusion, Ag-coated ZnO multilayer thin films were produced at 600 °C by PLD on fused quartz substrates by pulsed laser deposition. Utilizing several analytical techniques, the produced thin films' physicochemical characteristics were examined in order to learn more about their optical, structural, and morphological characteristics. These methods demonstrated the crystalline structure, excellent homogeneity surface, and production of structural particle size in both produced films. When the prepared thin films from the ZnO structure and the ZnO/Ag multilayer thin film structure were studied as a catalytic degradation material for the degradation of 4-nitrophenol, it was discovered that the degradation efficiency was greater than 90% after passing approximately 55 and 25 min, respectively.
Data availability
We confirm that we have known the research data policy, and the data are available.
References
F.S. Alamro, A.M. Mostafa, K.A.A. Al-Ola, H.A. Ahmed, A. Toghan, Synthesis of Ag nanoparticles-decorated CNTs via laser ablation method for the enhancement the photocatalytic removal of naphthalene from water. J. Nanomater. 11(8), 2142 (2021). https://doi.org/10.3390/nano11082142
E.A. Mwafy, A.M. Mostafa, Efficient removal of Cu (II) by SnO2/MWCNTs nanocomposite by pulsed laser ablation method. Nano-Struct. Nano-Objects 24, 100591 (2020). https://doi.org/10.1016/j.nanoso.2020.100591
A.M. Mostafa, E.A. Mwafy, The effect of laser fluence for enhancing the antibacterial activity of NiO nanoparticles by pulsed laser ablation in liquid media. Environ. Nanotechnol. Monit. Manag. 14, 100382 (2020). https://doi.org/10.1016/j.enmm.2020.100382
A.M. Mostafa, The enhancement of nonlinear absorption of Zn/ZnO thin film by creation oxygen vacancies via infrared laser irradiation and coating with Ag thin film via pulsed laser deposition. J. Mol. Struct. 1226, 129407 (2021). https://doi.org/10.1016/j.molstruc.2020.129407
A.M. Mostafa, Preparation and study of nonlinear response of embedding ZnO nanoparticles in PVA thin film by pulsed laser ablation. J. Mol. Struct. 1223, 129007 (2021). https://doi.org/10.1016/j.molstruc.2020.129007
E.A. Mwafy, A.M. Mostafa, N.S. Awwad, H.A. Ibrahium, Catalytic activity of multi-walled carbon nanotubes decorated with tungsten trioxides nanoparticles against 4-nitrophenol. J. Phys. Chem. Solids 158, 110252 (2021). https://doi.org/10.1016/j.jpcs.2021.110252
A.M. Mostafa, E.A. Mwafy, N.S. Awwad, H.A. Ibrahium, Synthesis of multi-walled carbon nanotubes decorated with silver metallic nanoparticles as a catalytic degradable material via pulsed laser ablation in liquid media. Colloids Surf. A: Physicochem. Eng. Asp. 626, 126992 (2021). https://doi.org/10.1016/j.colsurfa.2021.126992
A.M. Mostafa, E.A. Mwafy, Effect of dual-beam laser radiation for synthetic SnO2/Au nanoalloy for antibacterial activity. J. Mol. Struct. 1222, 128913 (2020). https://doi.org/10.1016/j.molstruc.2020.128913
H. Ali, A.M. Alsmadi, B. Salameh, M. Mathai, M. Shatnawi, N.M.A. Hadia, E.M.M., Ibrahim, Influence of nickel do** on the energy band gap, luminescence, and magnetic order of spray deposited nanostructured ZnO thin films. J. Alloys Compd. 816, 152538 (2020). https://doi.org/10.1016/j.jallcom.2019.152538
M.M. Rahman Khan, M. Akter, M.K. Amin, M. Younus, N. Chakraborty, Synthesis, luminescence and thermal properties of PVA–ZnO–Al2O3 composite films: towards fabrication of sunlight-induced catalyst for organic dye removal. J. Polym. Environ. 26, 3371–3381 (2018). https://doi.org/10.1007/s10924-018-1220-9
A.M. Alturki, Effect of preparation method on the particles size, dielectric constant and antibacterial properties of ZnO nanoparticles and thin film of ZnO/chitosan. Orient. J. Chem. 34, 548 (2018)
D.J. Edison, W. Nirmala, K.D.A. Kumar, S. Valanarasu, V. Ganesh, M. Shkir, S. AlFaify, Structural, optical and nonlinear optical studies of AZO thin film prepared by SILAR method for electro-optic applications. Physica B 523, 31–38 (2017). https://doi.org/10.1016/j.physb.2017.08.021
A.M. Khalil, S.H. Kenawy, Hybrid membranes based on clay-polymer for removing methylene blue from water. Acta Chim. Slov., 67(1), 96–104 (2020). https://doi.org/10.17344/acsi.2019.5227
F. Mousli, A.M. Khalil, F. Maurel, A. Kadri, M.M. Chehimi, Mixed oxide-polyaniline composite-coated woven cotton fabrics for the visible light catalyzed degradation of hazardous organic pollutants. Cellulose 27(13), 7823–7846 (2020). https://doi.org/10.1007/s10570-020-03302-7
Z. Ait-Touchente, A.M. Khalil, S. Simsek, S. Boufi, L.F.V. Ferreira, M.R. Vilar, R. Touzani, M.M. Chehimi, Ultrasonic effect on the photocatalytic degradation of Rhodamine 6G (Rh6G) dye by cotton fabrics loaded with TiO2. Cellulose 27(2), 1085–1097 (2020). https://doi.org/10.1007/s10570-019-02817-y
J. Omiri, Y. Snoussi, A.K. Bhakta, S. Truong, S. Ammar, A.M. Khalil, M. Jouini, M.M. Chehimi, Citric-acid-assisted preparation of biochar loaded with copper/nickel bimetallic nanoparticles for dye degradation. Colloid Interface 6(2), 18 (2022). https://doi.org/10.3390/colloids6020018
N.D. Jayram, S. Sonia, S. Poongodi, P.S. Kumar, Y. Masuda, D. Mangalaraj, N. Ponpandian, C. Viswanathan, Superhydrophobic Ag decorated ZnO nanostructured thin film as effective surface enhanced Raman scattering substrates. Appl. Surf. Sci. 355, 969–977 (2015). https://doi.org/10.1016/j.apsusc.2015.06.191
P. Suresh Kumar, A. Dhayal Raj, D. Mangalaraj, D. Nataraj, N. Ponpandian, L. Li, G. Chabrol, Growth of hierarchical based ZnO micro/nanostructured films and their tunable wettability behavior. Appl. Surf. Sci. 257, 6678–6686 (2011). https://doi.org/10.1016/j.apsusc.2011.02.101
J. Stryhalski, D.A. Duarte, L.M. Rebouta, J.C. Sagás, C.J. Tavares, L.C. Fontana, Nb-doped Ti2O3 films deposited through grid-assisted magnetron sputtering on glass substrate: electrical and optical analysis. Mater. Res. (2019). https://doi.org/10.1590/1980-5373-MR-2018-0524
A.M. Mostafa, E.A. Mwafy, V.F. Lotfy, A.H. Basta, Optical, electrical and mechanical studies of paper sheets coated by metals (Cu and Ag) via pulsed laser deposition. J. Mol. Struct. 1198, 126927 (2019). https://doi.org/10.1016/j.molstruc.2019.126927
A.M. Mostafa, V.F. Lotfy, E.A. Mwafy, A.H. Basta, Influence of coating by Cu and Ag nanoparticles via pulsed laser deposition technique on optical, electrical and mechanical properties of cellulose paper. J. Mol. Struct. 1203, 12772 (2020). https://doi.org/10.1016/j.molstruc.2019.127472
T.I. Zubar, L.V. Panina, N.N. Kovaleva, S.A. Sharko, D.I. Tishkevich, D.A. Vinnik, S.A. Gudkova, E.L. Trukhanova, E.A. Trofimov, S.A. Chizhik, S.V. Trukhanov, A.V. Trukhanov, Retracted article: anomalies in growth of electrodeposited Ni–Fe nanogranular films. CrystEngComm 20, 2306–2315 (2018). https://doi.org/10.1039/C8CE00310F
I. Kumar, A. Khare, Optical nonlinearity in nanostructured carbon thin films fabricated by pulsed laser deposition technique. Thin Sol. Films 611, 56–61 (2016). https://doi.org/10.1016/j.tsf.2016.05.023
A.M. Mostafa, E.A. Mwafy, Synthesis of ZnO/CdO thin film for catalytic degradation of 4-nitrophenol. J. Mol. Struct. 1221, 128872 (2020). https://doi.org/10.1016/j.molstruc.2020.128872
A.M. Mostafa, E.A. Mwafy, Laser-assisted for preparation Ag/CdO nanocomposite thin film: structural and optical study. Opt. Mater. 107, 110124 (2020). https://doi.org/10.1016/j.optmat.2020.110124
J.D. Pedarnig, J. Heitz, T. Stehrer, B. Praher, R. Viskup, K. Siraj, A. Moser, A. Vlad, M.A. Bodea, D. Bäuerle, N.H. Babu, D.A. Cardwell, Characterization of nano-composite oxide ceramics and monitoring of oxide thin film growth by laser-induced breakdown spectroscopy. Spectrochim. Acta. Part B 63, 1117–1121 (2008). https://doi.org/10.1016/j.sab.2008.06.012
N. Srinivasan, J. Kannan, S.J.I.J.C.T.R. Satheeskumar, Examination of antibacterial properties of pure and aluminum doped zinc oxide nanoparticles. Int. J. Chem. Tech. Res. 7, 1708–1712 (2015)
U.T. Khatoon, K.V. Rao, J.R. Rao, Y. Aparna, Synthesis and characterization of silver nanoparticles by chemical reduction method, In: International Conference on Nanoscience, Engineering and Technology (ICONSET 2011), IEEE, 2011, 97–99
M.M. ElFaham, M. Okil, A.M. Mostafa, Fabrication of magnesium metallic nanoparticles by liquid-assisted laser ablation. J. Opt. Soc. Am. B 37(9), 2620–2625 (2020). https://doi.org/10.1364/JOSAB.398543
E.A. Mwafy, A.M. Mostafa, Tailored MWCNTs/SnO2 decorated cellulose nanofiber adsorbent for the removal of Cu (II) from waste water. Radiat. Phys. Chem. 177, 109172 (2020). https://doi.org/10.1016/j.radphyschem.2020.109172
A.M. Mostafa, E.A. Mwafy, N.S. Awwad, H.A. Ibrahium, Linear and nonlinear optical studies of Ag/Zn/ZnO nanocomposite thin film prepared by pulsed laser deposition technique. Radiat. Phys. Chem. 179, 109233 (2021). https://doi.org/10.1016/j.radphyschem.2020.109233
M. Ahmad, I. Ahmad, E. Ahmed, M.S. Akhtar, N.J.J.o.M.L. Khalid, Facile and inexpensive synthesis of Ag doped ZnO/CNTs composite: Study on the efficient photocatalytic activity and photocatalytic mechanism. J. Mol. Liq. 311, 113326 (2020)
A. Pimentel, A. Araújo, B. Coelho, D. Nunes, M. Oliveira, M. Mendes, H. Águas, R. Martins, E. Fortunato, 3D ZnO/Ag surface-enhanced Raman scattering on disposable and flexible cardboard platforms. Materials 10, 1351 (2017)
P. Fageria, S. Gangopadhyay, S. Pande, Synthesis of ZnO/Au and ZnO/Ag nanoparticles and their photocatalytic application using UV and visible light. RSC Adv. 4, 24962–24972 (2014)
C. Ren, B. Yang, M. Wu, J. Xu, Z. Fu, T. Guo, Y. Zhao, C. Zhu, Synthesis of Ag/ZnO nanorods array with enhanced photocatalytic performance. J. hazard. Mater. 182, 123–129 (2010)
K. Bahedi, M. Addou, M. El Jouad, Z. Sofiani, M. Alaoui Lamrani, T. El Habbani, N. Fellahi, S. Bayoud, L. Dghoughi, B. Sahraoui, Z. Essaïdi, Diagnostic study of the roughness surface effect of zirconium on the third-order nonlinear-optical properties of thin films based on zinc oxide nanomaterials. Appl. Surf. Sci. 255, 4693–4695 (2009). https://doi.org/10.1016/j.apsusc.2008.11.045
S. Zhao, Y. Zhou, K. Zhao, Z. Liu, P. Han, S. Wang, W. **ang, Z. Chen, H. Lü, B. Cheng, Violet luminescence emitted from Ag-nanocluster doped ZnO thin films grown on fused quartz substrates by pulsed laser deposition. Physica B 373, 154–156 (2006)
H. Lin, T. Li, B.J. Janani, A. Fakhri, Fabrication of Cu2MoS4 decorated WO3 nano heterojunction embedded on chitosan: Robust photocatalytic efficiency, antibacterial performance, and bacteria detection by peroxidase activity. J. Photochem. Photobiol. B Biology 226, 112354 (2022). https://doi.org/10.1016/j.jphotobiol.2021.112354
A.S. Altowyan, A. Toghan, H.A. Ahmed, R.A. Pashameah, E.A. Mwafy, S.H. Alrefaee, A.M. Mostafa, Removal of methylene blue dye from aqueous solution using carbon nanotubes decorated by nickel oxide nanoparticles via pulsed laser ablation method. Radiat. Phys. Chem. 198, 110268 (2022). https://doi.org/10.1016/j.radphyschem.2022.110268
G. Zeng, H. You, M. Du, Y. Zhang, Y. Ding, C. Xu, B. Liu, B. Chen, X. Pan, Enhancement of photocatalytic activity of TiO2 by immobilization on activated carbon for degradation of aquatic naphthalene under sunlight irradiation. Chem. Eng. J. 412, 128498 (2021). https://doi.org/10.1016/j.cej.2021.128498
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research was financed by Egypt’s National Research Center (NRC) (Project No. 12020307).
Author information
Authors and Affiliations
Contributions
All authors share the same contributions for making this article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mostafa, A.M., Mwafy, E.A., Khalil, A.M. et al. ZnO/Ag multilayer for enhancing the catalytic activity against 4-nitrophenol. J Mater Sci: Mater Electron 34, 300 (2023). https://doi.org/10.1007/s10854-022-09631-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-022-09631-6