Abstract
Purpose
Contrast-enhanced ultrasound (CEUS) is a highly specific and sensitive method for assessing hemodynamically stable patients with blunt abdominal trauma. We evaluated the efficacy of CEUS in assessing renal trauma in different states of hemodynamic instability or shock.
Methods
Hemorrhagic renal lesions reflecting grade III–IV trauma were established in the kidneys of 25 mongrel dogs. Mild, moderate, and severe systemic hypotension was induced by controlled exsanguination. The features of renal trauma in CEUS and contrast-enhanced computed tomography (CECT) were assessed and compared before shock and during shock progression.
Results
Gross pathology showed that with trauma, the kidneys gradually shrank and became soft, and the active bleeding in the area of the renal trauma gradually reduced and stopped. No significant differences were observed in the trauma detection rates between CEUS and CECT at any stage of shock. During the baseline and mild shock stage, sonograms obtained after intravenous injection of contrast agent showed marked contrast medium extravasation and pooling at the site of active bleeding. With shock progression, the difference in enhancement between trauma areas and the surrounding renal tissue decreased: the trauma areas became indistinct and the abnormal enhancement associated with active bleeding diminished. Further, CEUS enabled visualization of changes in renal perfusion associated with shock progression. Changes in contrast agent arrival time and the time to peaking were observed earliest in the mild shock model. The contrast agent peak intensity reduced, while the washout time increased as shock progressed from moderate to severe.
Conclusion
In our canine model, CEUS was found to be as accurate as CECT in assessing hemorrhagic renal lesions. Thus, CEUS seems a promising tool for monitoring hemodynamic changes and predicting early shock to enable the conservative treatment of severe renal trauma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The kidney, a retroperitoneal organ with abundant blood flow and a fragile structure, is very vulnerable to external trauma. Renal trauma occurs in 8–10 % of abdominal trauma cases and 1–5 % of all trauma cases [1]. With the development of medical imaging methods and accumulating experience in this regard, conservative treatment for patients with renal trauma is increasingly being performed to maximally preserve renal tissue and function [2]. However, despite considerable advancements in abdominal trauma imaging, noninvasive imaging methods are still urgently needed at present.
The most common imaging methods for the assessment of renal trauma are ultrasound (US) imaging, contrast-enhanced computed tomography (CECT), magnetic resonance imaging (MRI), digital subtraction angiography, and radionuclide scintigraphy. Among these methods, CECT is the principal method used to determine the location and degree of abdominal viscera trauma, and it is considered as the gold standard for clinical assessment of renal trauma [3]. CECT is moderately helpful in the evaluation of the type and severity of parenchymal injury, the extent of perirenal hemorrhage and parenchymal devascularization, and the presence of urinary extravasation [4]. However, this method cannot be used for patients who are allergic to iodine contrast agents or for those who are hemodynamically unstable; moreover, it is not suitable for portable use because it involves large devices and the corresponding support facilities.
US imaging is an important diagnostic tool for abdominal trauma. It can be used for patients with unstable hemodynamics and can be safely used repeatedly with or without contrast agents [5]. Technological progress, including the development and application of new contrast agents, has made contrast-enhanced US (CEUS) as effective a tool as CECT for the diagnosis of abdominal trauma, monitoring of disease progression, and prediction of outcomes [5]. Compared to conventional US, CEUS with real-time, dynamic observation provides markedly better imaging rates, sensitivity, and specificity for the assessment of abdominal trauma [6–9]. As blood pool agents, contrast agents comprise microbubbles that do not leave the blood vessels easily. They are therefore not subjected to renal filtration or excretion and consequently behave as vascular tracers. Since new US contrast agents have good stability and compressive resistance, they can be used in CEUS for around-the-clock evaluation of organ tissue perfusion and treatment responses [8]. Compared to CT scanning, CEUS is also more suitable for bedside examinations and use in intensive care units [10].
The aim of the present study was to evaluate the efficacy of CEUS in assessing renal trauma in dogs with hemorrhagic shock. An animal model with different blood pressure states was used to mimic various degrees of hemorrhagic shock. Through our findings, we hoped to provide diagnostic information that may be useful in the conservative treatment of renal trauma.
Materials and methods
Animals
All experiments were conducted according to the guidelines of the National Institute of Health for the Care of Laboratory Animals, and the protocol was approved by the Institutional Animal Care and Use Committee.
A total of 25 healthy male mongrel dogs with a health certificate (license number: SYXK 2008-0009, Bei**g) were used (age 2–3 years; weight 16.5 ± 1.5 kg). All animals had a 3-day adaptive period prior to experimentation, followed by a 12-h period of preoperative fasting, with free access to water.
For anesthesia, intravenous access was established in the forelimbs of dogs, through which 3 % pentobarbital sodium (30 mg/kg body weight; Sinopharm Chemical Reagent, China) was slowly injected. To enable tracheal intubation, the dogs were placed in the supine position on the manipulation platform. A ventilator was connected to the tracheal tube for mechanical ventilation. Pentobarbital was intermittently administered intravenously to maintain the anesthesia. Heparin (300 IU/kg; Guangzhou Baiyun Pharmaceutical, China) was injected intravenously to induce systemic heparinization. The right femoral artery was cannulated, and vital signs were recorded using a cardiogram monitor (model PEG-1000c; Nihon Kohden, Japan) linked to a pressure sensor at the end of the cannulation tube. The left femoral artery was cannulated for blood sampling. Blood oxygen saturation (SaO2) was monitored at the front of the dogs’ tongues. A bladder catheter was used to record the urine volume.
Animal shock model
The same physician performed all the laparotomies and constructed the models of kidney trauma and shock. The abdominal skin was cut open under aseptic conditions, and the left kidney was fully exposed via a left abdominal incision. A cross incision with a relatively jagged cutting edge, 1.0–2.0 cm in both transverse and longitudinal lengths, was made in the kidney with a small scalpel, without injuring the blood vessels in the renal hilum. The incisions were categorized as grade III–IV renal trauma according to the classification standards of the American Association for the Surgery of Trauma [11].
The left femoral artery was used for intermittent bloodletting, at a rate of 0.5 mL kg−1 min−1 (~8 mL/min). The left femoral vein was used for transfusion. In this study, we used an autologous blood reclaiming device (Model 3000P; Bei**g **g**g Medical Equipment Co., Ltd., China). A specific level of hypotension (mild/moderate/severe systemic hypotension) was induced by controlled exsanguination. Additional amounts of blood were transfused or exsanguinated, as indicated by blood pressure measurements, to maintain stable blood pressure for each grade of shock. Blood pressure grade was classified into four states: stable blood pressure [normal mean arterial pressure (MAP)], mild hypotension (70 % MAP), moderate hypotension (50 % MAP), and severe hypotension (40 % MAP) [12]. The entire experimental procedure lasted approximately 7 h.
Image acquisition
After the incision was made and at each stage of shock (mild, moderate, and severe hypotension), conventional US and CEUS were performed with a CX50 system (Philips Medical Systems, Andover, MA) and a L12-3 transducer (3.0–12 MHz). After conventional US examination was performed on each dog, a bolus of SonoVue™ (0.025 mL/kg; Bracco, Italy) was rapidly administered through the accessory cephalic vein. Immediately after this injection, the dog was continuously scanned for 3–5 min. For CEUS, the pulse inversion harmonic and energy-modulated technique at low acoustic power (mechanical index 0.07) was used to detect the strong nonlinear fundamental component and the nonlinear second-harmonic response. In this process, the signal-to-noise ratio was increased by 15–20 dB, and thus a much stronger contrast signal was produced. The pulse inversion harmonic and energy-modulated technique was also used to visualize the sonographic appearance of the lesions. The scan settings (gain, scanning depth, and time gain control) were optimized independently for each region. Digital images were recorded as single-frame pictures and multiple cine loops and saved for offline analysis.
Both US and CEUS investigations were conducted by the same operator. For CEUS, kidney trauma was defined as the absence of enhancement or a low-enhanced perfusion defect in the lesion. Active hemorrhage was defined as outflow or increased uptake of the contrast agent in and around the lesion. QLAB software (Philips Medical Systems, Andover, MA), provided with the US instrument, was used for analysis. Multi-points, with 1-cm spacing from the center of the trauma, were selected as regions of interest in the renal cortex. Using contrast agent injection as the zero time point, ultrasonic imaging perfusion measurements were recorded, including arrival time (AT), time to peak (TTP), peak intensity (PI), and washout time (WT). After completion of CEUS studies, all images were independently analyzed by two experts.
Conventional CT and enhanced CT scans (with GE Lightspeed 16 type or Sensation 64-layer spiral CT scanner; GE Medical Systems, Milwaukee, WI) were performed from the epigastrium to the lower edge of kidney. The scans were initially obtained with 10-mm layer thickness and then with 3- or 1-mm layer thickness. Ioversol (2 mL/kg) was used for the enhanced CT. CT slices were read and diagnosed by trained doctors.
Statistical analysis
Statistical analyses were performed with SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). Most data were presented as the mean ± SD. Data from experiments were analyzed using the least significant difference (LSD) test or chi-square test as appropriate. Statistical significance was defined as a P value < 0.05.
Results
Hemorrhagic shock model
Twenty-three dogs remained alive at the end of the experiment, and two died of severe shock. Physiological data for each stage of shock are presented in Table 1. Progressive hypotension, MAP, oxygen saturation, and urine volume decreased, while HR increased significantly with progressive shock (P < 0.05).
Gross pathology showed that 10 dogs had incurred grade III renal trauma, and 15 had incurred grade IV trauma. Prior to shock, all kidneys were fully functional and flexible. With the onset of shock, the kidneys became smaller (Fig. 1) and softer and had a pale cortex and dark red medulla; further, the cortical vessels became constricted and less flexible.
Changes in CEUS perfusion features with shock progression
In the pre-shock state, CEUS PI was rapidly observed in the interlobar artery, arcuate arteries, renal cortex, and medulla after injection of the ultrasound contrast agents, and the values then returned to the baseline levels during 9.63 ± 0.41 s. With the progression of shock, the PI declined slowly, while the TTP and WT increased. During mild shock, AT and TTP exhibited the earliest changes, and these changes became more pronounced with increasing degrees of shock. In cases of moderate and severe shock, PI began to decline, while WT became significantly prolonged (Fig. 2a–d).
CEUS imaging characteristics of kidneys with progression of shock
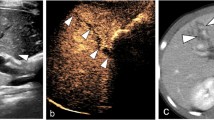
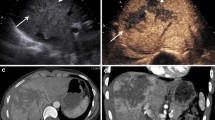
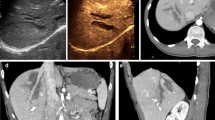
Gross pathology, CEUS, and CECT images of grade III renal trauma with active bleeding at baseline are shown in Fig. 3a–c and in mild shock are depicted in Fig. 3d–f. Figure 3g–i shows images of grade III renal trauma with active bleeding in moderate shock and Fig. 3j–l is images for grade IV renal trauma with active bleeding in severe shock.
(a–c) Gross pathology, CEUS, and CECT images of grade III renal trauma with active bleeding (yellow arrow) at baseline; (d–f) gross pathology, CEUS, and CECT images of grade III renal trauma with active bleeding (yellow arrow) in mild shock; (g–i) gross pathology, CEUS, and CECT images of grade III renal trauma with active bleeding (yellow arrow) in moderate shock; (j–k) gross pathology, CEUS, and CECT images of grade III renal trauma with active bleeding (yellow arrow) in severe shock. CEUS contrast-enhanced ultrasonography, CECT contrast-enhanced computed tomography
Before shock, the kidneys were brightly colored, with an intensive cortical vascular distribution and a steady pulse (Fig. 3a). Overall, 76 % (19/25) of the dogs showed renal trauma on conventional US, but accurate classification was not possible. In contrast, CEUS showed constriction of the renal arteries within the trauma region at all stages of shock, with the region of trauma being either not enhanced or only weakly enhanced. Trauma diagnosis by CEUS was 80 % accurate for grade III (8/10) and 100 % accurate for grade IV (15/15) traumatic incisions. During active bleeding, visible contrast agent flowing out from the rupture in the renal capsule was considered to indicate either no or low contrast enhancement (Fig. 3c).
Gross examination showed no bleeding in any of the 25 wounds during the stage of severe shock (Fig. 3j). CEUS showed that with progressive shock, the flow of the contrast agent slowed significantly, and arteries at all depths became more constricted. The contrast agent slowly filled the renal capsule, accumulated in the vascular endings, and dispersed, resulting in reduced intensity of contrast enhancement and extended WT. During the stage of severe shock, perfusion of the renal parenchyma declined, and the intensity of contrast enhancement decreased. The diagnostic sensitivity of CEUS declined to 60 % for grade III (6/10) and 80 % for grade IV trauma (12/15) (Fig. 3e, h, k).
Comparison of CEUS and CECT results
On CECT examination, the renal lesion appeared as a low attenuation area when the kidney capsule was incomplete, and active bleeding appeared as high attenuation areas (Fig. 3c). During the baseline and mild shock stage, sonograms obtained after the intravenous injection of contrast agent showed marked contrast medium extravasation and pooling at the site of active bleeding (Fig. 3b, e). Graded hypotension resulted in a significant, stepwise diminution in renal blood flow on both CEUS and CECT. As shown in Table 2, no significant difference was found in the shock detection rate between CEUS and CECT during any stage of shock (P > 0.05). CECT imaging showed kidney shrinkage, with a decrease in both the areas of enhancement and irregular non-enhanced areas (Fig. 3f, i, l).
Discussion
The major finding of this study was that CEUS had detection rates of renal trauma similar to those of CECT during various stages of shock. CEUS also indicated active bleeding in the traumatic lesions, which appeared as contrast medium extravasation or pooling, both inside and outside the renal capsule, which was consistent with the findings of previous animal experiments and clinical studies [7].
Unlike contrast agents for CT, MRI, and radionuclide scintigraphy, microbubble ultrasound contrast agents remain in the blood and are not cleared by permeation into interstitial spaces or by glomerular filtration and renal tubular transport. Further, US contrast agents have blood flow dynamics similar to those of red blood cells and are therefore ideal erythrocyte tracers [13]. Compared to CT and MR perfusion imaging, CEUS has the advantages of showing the perfusion of parenchymal organs, simplicity, and a short data acquisition time. CEUS can also provide real-time data on organ tissue perfusion at the microcirculatory level and reveal the normal physiology and pathological anatomy of and pathophysiological changes in human tissues and organs. In addition, CEUS imaging can be performed repeatedly with no risk of radiation injury, renal toxicity, or serious adverse reactions. Thus, CEUS is suitable for critically ill patients and those who require repeated imaging [14, 15].
Shock causes a series of physiological reactions, including a sharp reduction in renal blood flow, reduced glomerular filtration rate, intracellular acidosis, adenosine triphosphate consumption, and enhanced activity of the renin–angiotensin–aldosterone system. This cascade leads to contraction of the renal vascular bed and release of catecholamines into the blood [16, 17]. Renal cortical blood vessels are abundant in sympathetic fibers with adrenaline receptors, which are particularly sensitive to catecholamines. After injection of a US contrast agent, time–intensity curves can be calculated to quantitate microcirculation perfusion [17]. In the present study, we found that the AT and TTP after injection of the contrast agents were delayed at various stages of shock, possibly because the contrast medium was obstructed by renal vascular contraction. However, PI did not change significantly during mild shock, suggesting that it is not a sensitive indicator. During early shock, renal cortical perfusion is maintained to guarantee the function of other important organs such as the heart and brain [18]. With the progression of ischemia, the high secretion of angiotensin and endothelin would cause renal tissue damage but could also increase afferent and efferent arteriolar resistance. As a result, renal cortical blood perfusion decreases progressively, the number of microbubbles in the renal cortex is reduced, and the reflection backscatter signal deceases. Correspondingly, cortex echo intensity is significantly decreased. An important observation of the present study was the decreased PI with increased AT and TTP with shock progression. Moreover, WT was prolonged, suggesting that the capillary bed was constricted. These manifestations can help to predict the occurrence and progression of hemorrhagic shock. On the other hand, they may also affect the evaluation of traumatic lesions by CEUS because they can lead to reduced contrast between the traumatic lesion and the surrounding splenic tissue.
Our study has some limitations. First, the shock model cannot completely mimic the blood flow dynamics in human trauma patients as they are not anesthetized, which can affect physiological indices. Second, the systemic heparinization administered to the dogs in this study is not directly translatable to clinical settings. Third, our experiments were conducted with a relatively simple model, and CEUS needs to be evaluated in the complex setting of human trauma. Finally, CEUS is still a new method for the analysis of blood flow perfusion using time–intensity curves. Several issues in this technique remain to be evaluated and standardized, including the depth of detection; appropriate contrast agent dose; effects of respiratory movement; and instrument settings, including depth of focus, time gain compensation, total gain, and mechanical indexes.
In conclusion, CEUS has certain advantages in the evaluation of different grades of renal trauma. In our canine model, CEUS and CECT showed similar detection rates for grade III–IV renal trauma during various stages of shock. It appears that CEUS is a promising tool for monitoring hemodynamic changes and predicting early shock to enable the conservative treatment of severe renal trauma.
References
Lobo SM, Salgado PF, Castillo VG, et al. Effects of maximizing oxygen delivery on morbidity and mortality in high-risk surgical patients. Crit Care Med. 2000;28:3396–404.
Diederichs W, Mutze S. Renal trauma: is open surgery still up to date? Urologe A. 2003;42:322–7.
Nast-Kolb D, Bail HJ, Taeger G. Current diagnostics for intra-abdominal trauma. Chirurg. 2005;76:919–26.
Pasławski M, Kołtyś W, Złomaniec J, et al. Imaging diagnostics of renal trauma. Ann Univ Mariae Curie Sklodowska Med. 2004;59:328–34.
Akgur FM, Aktug T, Olguner M, et al. Prospective study investigating routine usage of ultrasonography as the initial diagnostic modality for the evaluation of children sustaining blunt abdominal trauma. J Trauma. 1997;42:626–8.
Tang J, Li W, Lv F, et al. Comparison of gray-scale contrast-enhanced ultrasonography with contrast-enhanced computed tomography in different grading of blunt hepatic and splenic trauma: an animal experiment. Ultrasound Med Biol. 2009;35:566–75.
Thorelius L. Emergency real-time contrast-enhanced ultrasonography for detection of solid organ injuries. Eur Radiol. 2007;17:F107–11.
Catalano O, Sandomenico F, Raso MM, et al. Real-time, contrast-enhanced sonography: a new tool for detecting active bleeding. J Trauma. 2005;59:933–9.
Sato M, Yoshii H. Reevaluation of ultrasonography for solid-organ injury in blunt abdominal trauma. J Ultrasound Med. 2004;23:1583–96.
Schneider A, Johnson L, Goodwin M, et al. Bench-to-bedside review: contrast enhanced ultrasonography—a promising technique to assess renal perfusion in the ICU. Crit Care. 2011;15:157.
Moore EE, Cogbill TH, Jurkovich GJ, et al. Organ injury scaling: spleen and liver (1994 revision). J Trauma. 1995;38:323–4.
Taylor GA, Barnewolt CE, Adler BH, et al. Renal cortical ischemia in rabbits revealed by contrast-enhanced power Doppler sonography. AJR Am J Roentgenol. 1998;170:417–22.
Wei K, Jayaweera AR, Firoozan S, et al. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. 1998;97:473–83.
Quaia E. Microbubble ultrasound contrast agents: an update. Eur Radiol. 2007;17:1995–2008.
Hoeffel C, Mule S, Huwart L, et al. Renal blood flow quantification in pigs using contrast-enhanced ultrasound: an ex vivo study. Ultraschall Med. 2010;31:363–9.
Tiefenthaler M, Riedl-Huter C. Value of sonography in kidney transplantation. Acta Med Austriaca. 2001;28:74–7.
Feingold S, Gessner R, Guracar IM, et al. Quantitative volumetric perfusion map** of the microvasculature using contrast ultrasound. Invest Radiol. 2010;45:669–74.
Xu HX. Contrast-enhanced ultrasound: the evolving applications. World J Radiol. 2009;1:15–24.
Acknowledgments
The study was supported by National Natural Science Foundation of China (No. 81071279).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
All experiments were conducted according to the guidelines issued by the National Institute of Health for the Care of Laboratory Animals and according to a protocol approved by our Institutional Animal Care and Use Committee.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Lin, Q., Lv, F., Luo, Y. et al. Contrast-enhanced ultrasound for evaluation of renal trauma during acute hemorrhagic shock: a canine model. J Med Ultrasonics 42, 199–205 (2015). https://doi.org/10.1007/s10396-014-0601-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-014-0601-5