Abstract
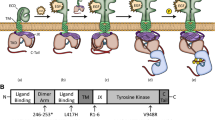
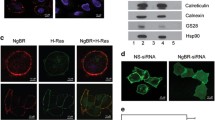
Ligand-dependent or independent oligomerization of receptor protein tyrosine kinase (RPTK) is often an essential step for receptor activation and intracellular signaling. The novel oncogene with kinase-domain (NOK) is a unique RPTK that almost completely lacks an ectodomain, expresses intracellularly and activates constitutively. However, it is unknown whether NOK can form oligomer or what function oligomerization would have. In this study, two NOK deletion mutants were generated by either removing the ectodomain (NOKΔECD) or including the endodomain (NOK-ICD). Co-immunoprecipitation demonstrated that the transmembrane (TM) domain of NOK was essential for its intermolecular interaction. The results further showed that NOK aggregated more closely as lower order oligomers (the dimer- and trimer-sized) than either deletion mutant did since NOK could be cross-linked by both Sulfo-EGS and formaldehyde, whereas either deletion mutant was only sensitive to Sulfo-EGS. Removing the NOK TM domain (NOK-ICD) not only markedly promoted higher order oligomerization, but also altered the subcellular localization of NOK and dramatically elevated the NOK-mediated constitutive activation of extracellular signal-regulated kinase (ERK). Moreover, NOK-ICD but not NOK or NOKΔECD was co-localized with the upstream signaling molecule RAS on cell membrane. Thus, TM-mediated intermolecular contacting may be mainly responsible for the constitutive activation of NOK and contribute to the autoinhibitory effect on RAS/MAPK signaling.
Similar content being viewed by others
References
Amachika, T., Kobayashi, D., Moriai, R., Tsuji, N., and Watanabe, N. (2007). Diagnostic relevance of overexpressed mRNA of novel oncogene with kinase-domain (NOK) in lung cancers. Lung Cancer 56, 337–340.
Bartkiewicz, M., Houghton, A., and Baron, R. (1999). Leucine zipper-mediated homodimerization of the adaptor protein c-Cbl. A role in c-Cbl’s tyrosine phosphorylation and its association with epidermal growth factor receptor. J. Biol. Chem. 274, 30887–30895.
Blume-Jensen, P., and Hunter, T. (2001). Oncogenic kinase signalling. Nature 411, 355–365.
Brennan, P.J., Kumagai, T., Berezov, A., Murali, R., and Greene, M.I. (2000). HER2/neu: mechanisms of dimerization/oligomerization. Oncogene 19, 6093–6101.
Chen, Y., Li, Y.H., Chen, X.P., Gong, L.M., Zhang, S.P., Chang, Z.J., Zhang, X.F., Fu, X.Y., and Liu, L. (2005). Point mutation at single tyrosine residue of novel oncogene NOK abrogates tumorigenesis in nude mice. Cancer Res 65, 10838–10846.
Clayton, A.H., Walker, F., Orchard, S.G., Henderson, C., Fuchs, D., Rothacker, J., Nice, E.C., and Burgess, A.W. (2005). Ligand-induced dimer-tetramer transition during the activation of the cell surface epidermal growth factor receptor-A multidimensional microscopy analysis. J. Biol. Chem. 280, 30392–30399.
Fan, P.D., Cong, F., and Goff, S.P. (2003). Homo- and heterooligomerization of the c-Abl kinase and Abelson-interactor-1. Cancer Res. 63, 873–877.
Guy, P.M., Platko, J.V., Cantley, L.C., Cerione, R.A., and Carraway, K.L., 3rd. (1994). Insect cell-expressed p180erbB3 possesses an impaired tyrosine kinase activity. Proc. Natl. Acad. Sci. USA 91, 8132–8136.
Hubbard, S.R. (2002). Autoinhibitory mechanisms in receptor tyrosine kinases. Front Biosci. 91, d330–340.
Jiang, G., and Hunter, T. (1999). Receptor signaling: When dimerization is not enough. Curr. Biol. 9, R568–R571.
Kani, K., Warren, C.M., Kaddis, C.S., Loo, J.A., and Landgraf, R. (2005). Oligomers of ERBB3 have two distinct interfaces that differ in their sensitivity to disruption by heregulin. J. Biol. Chem. 280, 8238–8247.
Kimbro, K.S., Duschene, K., Willard, M., Moore, J.A., and Freeman, S. (2008). A novel gene STYK1/NOK is upregulated in estrogen receptor-alpha negative estrogen receptor-beta positive breast cancer cells following estrogen treatment. Mol. Biol. Rep. 35, 23–27.
Li, E., and Hristova, K. (2006). Role of receptor tyrosine kinase transmembrane domains in cell signaling and human pathologies. Biochemistry 45, 6241–6251.
Li, Y.H., Zhong, S., Rong, Z.L., Ren, Y.M., Li, Z.Y., Zhang, S.P., Chang, Z., and Liu, L. (2007). The carboxyl terminal tyrosine 417 residue of NOK has an autoinhibitory effect on NOK-mediated signaling transductions. Biochem. Biophys. Res. Commun. 356, 444–449.
Li, Y.H., Chang, Z.J., and Liu, L. (2008a). NOK interacts with Akt and enhances its activation. Prog. Biochem. Biophys. 35, 29–34.
Li, Y.H., Rong, Y., Chang, Z J., and Liu, L. (2008b). NOK activates STAT3 signaling by a JAK2-dependent mechanism. Prog. Biochem. Biophys. 35, 143–150.
Liu, L., Yu, X.Z., Li, T.S., Song, L.X., Chen, P.L., Suo, T.L., Li, Y.H., Wang, S.D., Chen, Y., Ren, Y.M., et al. (2004). A novel protein tyrosine kinase NOK that shares homology with platelet- derived growth factor/fibroblast growth factor receptors induces tumorigenesis and metastasis in nude mice. Cancer Res. 64, 3491–3499.
Manning, G., Whyte, D.B., Martinez, R., Hunter, T., and Sudarsanam, S. (2002). The protein kinase complement of the human genome. Science 298, 1912–1934.
McWhirter, J.R., Galasso, D.L., and Wang, J.Y. (1993). A coiled-coil oligomerization domain of Bcr is essential for the transforming function of Bcr-Abl oncoproteins. Mol. Cell. Biol. 13, 7587–7595.
Mohammadi, M., Honegger, A., Sorokin, A., Ullrich, A., Schlessinger, J., and Hurwitz, D.R. (1993). Aggregationinduced activation of the epidermal growth factor receptor protein tyrosine kinase. Biochemistry 32, 8742–8748.
Moriai, R., Kobayashi, D., Amachika, T., Tsuji, N., and Watanabe, N. (2006). Diagnostic relevance of overexpressed NOK mRNA in breast cancer. Anticancer Res. 26, 4969–4973.
Ozaki, K., Miyazaki, S., Tanimura, S., and Kohno, M. (2005). Efficient suppression of FGF-2-induced ERK activation by the cooperative interaction among mammalian Sprouty isoforms. J. Cell Sci. 118, 5861–5871.
Pluk, H., Dorey, K., and Superti-Furga, G. (2002). Autoinhibition of c-Abl. Cell 108, 247–259.
Robinson, D.R., Wu, Y.M., and Lin, S.F. (2000). The protein tyrosine kinase family of the human genome. Oncogene 19, 5548–5557.
Schlessinger, J. (2002). Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell 110, 669–672.
Simpson, J.C., Wellenreuther, R., Poustka, A., Pepperkok, R., and Wiemann, S. (2000). Systematic subcellular localization of novel proteins identified by large-scale cDNA sequencing. EMBO Rep. 1, 287–292.
Smith, K.M., Yacobi, R., and Van Etten, R.A. (2003). Autoinhibition of Bcr-Abl through its SH3 domain. Mol. Cell 12, 27–37.
Tong, Q., **ng, S., and Jhiang, S.M. (1997). Leucine zipper-mediated dimerization is essential for the PTC1 oncogenic activity. J. Biol. Chem. 272, 9043–9047.
Ye, X., Ji, C., Huang, Q., Cheng, C., Tang, R., Xu, J., Zeng, L., Dai, J., Wu, Q., Gu, S., et al. (2003). Isolation and characterization of a human putative receptor protein kinase cDNA STYK1. Mol. Biol. Rep. 30, 91–96.
Yu, X., Sharma, K.D., Takahashi, T., Iwamoto, R., and Mekada, E. (2002). Ligand-independent dimer formation of epidermal growth factor receptor (EGFR) is a step separable from ligand-induced EGFR signaling. Mol. Biol. Cell 13, 2547–2557.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Li, YH., Wang, YY., Zhong, S. et al. Transmembrane helix of novel oncogene with kinase-domain (NOK) influences its oligomerization and limits the activation of RAS/MAPK signaling. Mol Cells 27, 39–45 (2009). https://doi.org/10.1007/s10059-009-0003-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10059-009-0003-5