Abstract
The effect of Ca2+ − do** on the electrical conductivity of the oxide BaLa2In2O7 with Ruddlesden-Popper structure was investigated for the first time. The BaCaxLa2-xIn2O7-0.5× solid solution exists in the composition range of 0 ≤ x ≤ 0.10. The electrical conductivity of the BaCaxLa2-xIn2O7-0.5× ceramic samples was measured as a function of temperature (T), oxygen partial pressure (pO2), and water vapor partial pressure (pH2O). The introduction of Ca2+ into the crystal lattice led to an increase in oxygen-ion conductivity by 0.25 orders of magnitude at 500 °C, which is attributed to the formation of oxygen vacancies. The share of oxygen-ion transport increased from 23 to 80% with decreasing temperature from 900 to 425 °C, compared to 20% for the undoped phase for the same temperature range. The increase in pH2O increases the proton concentration in the structure, which is confirmed by the growth of conductivity and the reduction in activation energy. The activation energy of proton conduction increases with increasing x due to additional contribution of the proton «trap**» energies ΔEH. The doped BaCaxLa2-xIn2O7-0.5× samples are nearly pure protonic conductors below 600 °C, but undoped sample exhibits predominant proton transport below 300 °C. The electrical properties of BaMxLa2-xIn2O7-0.5x (M = Ca2+, Sr2+, and Ba2+) were compared.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Materials with the Ruddlesden–Popper (RP) structure have recently attracted significant attention as an alternative to the conventional perovskites due to relatively high oxygen-ion and proton conductivities and a possibility to apply them as electrolytes in the Intermediate Temperature Solid Oxide Fuel Cells (IT- SOFCs) [1,2,3].
The RP layered oxides have the general formula of An+1BnO3n+1 (typically n = 1, 2, and 3). The crystal structure of the RP phases consists of n consecutive perovskite layers ABO3, alternating with AO rock-salt-type layers, stacking along the crystallographic c-axis, as represented by the formula (AO)(ABO3)n. Such layered compounds can exhibit high oxygen-ion conductivity due to the possibility of oxygen-ion transport through both interstitial and vacancy mechanisms [4,5,6]. In addition, such phases can exhibit protonic transport due to incorporation of OH− groups into the AO rock-salt-type layers [7, 8].
The majority of studies on the electrical properties of An+1BnO3n+1 were performed for Ruddlesden-Popper materials with n = 1 [9]. Due to lattice flexibility, such phases are able to adapt a various types of dopants, the presence of which can significantly affect the electrical conductivity. In 2014, a new structural type of modular perovskite BaNdInO4 was described as an oxygen-ion conductor [10]. The structure consists of the oxide-ion conducting A-type rare earth oxide-like Nd2O3 layer and a perovskite-like [(Ba3/4Nd1/4)InO3]2 slab [11]. The systematic studies of oxygen-ion and proton transport have been reported for the BaLaInO4 RP-phase [12,13,14]. The structure consists of perovskite blocks (with the InO6 octahedra) separated by rock-salt-type layers composed of the (BaLa)O9 polyhedra [15]. It has been shown that the acceptor do** of BaLaInO4 by Ca2+, Sr2+, and Ba2+ led to an increase not only in oxygen-ion conductivity, but also in the proton conductivity [16]. The proton conductivity of doped samples increased in the order of Ca2+– Sr2+– Ba2+ – dopants. The donor-type do** can also increase the proton conductivity. For example, the proton conductivity of the BaLaIn0.9Ti0.1O4.05 and BaLaIn0.9Zr0.1O4.05 samples is ~ 0.8 orders of magnitude higher than that of the BaLaInO4 parent phase [16]. It should be concluded, that most studies on electrical properties were devoted to indates, which is explained by the ability of indium to adapt different coordination numbers upon do** and by the possibility of obtaining a wide variety of phases. Thus, the indates with the general composition of AIILanInnO3n+1 at n = 1 have been studied in detail as oxygen-ion and proton conductors. However, the available data on ionic transport for RP phases with n = 2 (two perovskite blocks) is scarce.
In 2001, Caldes et al. [17] obtained a new indate of the BaLa2In2O7 composition, which is a member of the Ruddlesden-Popper series An+1BnO3n+1 at n = 2. It was shown that the layered structure of BaLa2In2O7 consisted of rock-salt-type layers separated by two perovskite blocks. The BaLa2In2O7 oxide crystallized in a tetragonal cell with the unit cell parameters of a = 5.9141 Å, c = 20.8231 Å. The rock salt layers have smaller thickness (~ 2.76 Å) compared to that of the perovskite blocks (~ 3.08 Å), which indicates that they are mainly occupied by lanthanum [17]. Based on calculations, it was confirmed that the Ba2+ cations are located in the perovskite blocks, while La3+ – in the rock-salt-type layers [17].
Later in 2010, Tyitov et al. [18] performed synthesis and determined the crystal structure of the double-layered indates ALn2In2O7 (A = Ca, Sr, Ba; Ln = La, Nd). BaLn2In2O7 indates were synthesized by co-crystallization (evaporation with intensive stirring) of aqueous solutions of barium acetate, rare earth, and indium nitrates with a ratio of Ba:Ln:In = 1:2:2 followed by heat treatment of the resulting powders to remove nitrogen oxides. The crystal structure of BaLn2In2O7 belongs to the P42/mnm space group (sp. gr.) with the unit cell parameters of a = 5.9152 Å and c = 20.861 Å [18]. The distribution of barium and Ln atoms between the 4f and 8j crystallographic positions for BaLn2In2O7 was analyzed by calculating the sums of valence bonds [18]. The results indicate that barium cations are localized only in the perovskite block, and Ln cations are placed only in LnO9 polyhedra, which “stitch” adjacent perovskite blocks. Two O(2) oxygen atoms are located at significantly greater distances (0.351(1) − 0.356(2)) nm from the Ln atom, so the coordination number of Ln atoms can be considered as 7 + 2 [18].
Ionic conductivity (O2− and H+) of the double-layered RP phases have been studied recently, starting from 2022 [19,20,21,22,23,24,25]. It was shown that their ionic conductivity exceeds the conductivity of the single-layered phases (n = 1). The ionic (O2−, H+) conductivity of undoped La-containing phase BaLa2In2O7 was higher than the conductivity of the Nd-containing sample BaNd2In2O7 [19]. It was also shown that the protonic conductivity of Sc-containing sample SrLa2Sc2O7 was lower than for BaLa2In2O7 composition [20]. Therefore, the strategy of modifying phase BaLa2In2O7 is the most promising. Unlike the single-layered phase BaLaInO4, donor do** of double-layered phase BaLa2In2O7 was not effective. For example, the introduction of donor dopant Ti4+ into the In3+-sublattice led to a decrease in conductivity [21]. Acceptor do** with Sr2+ and Ba2+ was the most effective, which led to the increase in the conductivity values up to ~ 1.5 orders of magnitude [22, 23]. Isovalent do** was carried out by introducing Gd3+ into the La3+ sublattice of BaLa2In2O7. Gadolinium do** led to an increase in the conductivity values up to ~ 0.5 orders of magnitude [24].
Summarizing these results, we can conclude that La-containing phase BaLa2In2O7 is the most highly conductive among known undoped double-layered phases BaLn2In2O7 [25], and its acceptor do** allows the most significant increase in ionic conductivity. However, acceptor do** has been studied only for Sr2+ and Ba2+-substituted solid solutions. Further studies are necessary for an understanding of the ionic transport features in such phases. In particular, it is important to study the substitution of lanthanum with calcium and make a comparison with previously obtained data for other alkaline earth dopants.
In this paper, the calcium-doped solid solution BaCaxLa2-xIn2O7-0.5× with the RP structure was synthesized and investigated for the first time, transport properties of the BaCaxLa2-xIn2O7-0.5× have been studied at various temperatures and oxygen/water vapor partial pressures. The oxygen-ion and proton conductivities have been determined. It has been shown that calcium do**, leading to the formation of oxygen vacancies, can increase both oxygen-ion and proton conductivity, the increase reached 0.25 orders of magnitude at 500 °C. The do** also led to an increase in ionic transport numbers. The electrical properties of BaCaxLa2-xIn2O7-0.5× and the Sr2+/Ba2+-doped solid solutions are compared. The correlation between structural (lattice volume and lattice-free volume) and electrical properties is reported. The results demonstrate that the optimization of structural parameters allows to obtain electrolyte materials suitable for intermediate temperature proton conducting SOFCs.
Experimental
Solid-state synthesis
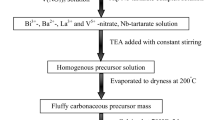
The compositions BaCaxLa2-xIn2O7-0.5x (where x = 0; 0.05; 0.10; 0.15; 0.20; 0.25; 0.30; 0.40; 0.50) were synthesized by the solid-state method. The starting materials BaCO3, CaCO3, La2O3, and In2O3 (REACHIM, RF, 99.99%) were used. Before weighting, heat treatment of the substances was carried out to remove adsorbed H2O (CaCO3 – 300 °C, BaCO3 and In2O3 – 500 °C); La2O3 was calcined at 1100 °C for 3 h to decompose the surface lanthanum carbonates LaOHCO3 and La2O2CO3. The stoichiometric amounts of oxides/carbonates were weighed on an analytical balance ACCULAB ALC-210d4 (Germany) with an accuracy of ± 0.0001 g. The powders were mixed and ground in an agate mortar with ethanol.
The synthesis was performed by annealing the powder mixtures at different temperatures in air with intermediate grinding in the following order: 700 °C (24 h); 800 °C (24 h); 900 °C (24 h); 1000 °C (24 h); 1100 °C (24 h). Phase composition was analyzed by using X-ray powder diffraction (XRPD), and the synthesis was considered to be completed when no traces of the starting reagents and/or any impurities were observed. The samples containing impurity phases were additionally annealed at 1200 °C for 24 h in air.
X-ray powder diffraction (XRPD)
Phase purity of the obtained samples was confirmed by XRPD at room temperature on a Bruker D8 Advance powder diffractometer (Germany) in monochromatic CuKα radiation over the 2θ range of 10–80° with a step of 0.02°. The Rietveld refinement was performed using Fullprof software.
Electrical conductivity measurements
To study the electrical properties the powder samples were pressed into pellets (10 mm in diameter with the thickness of 2–3 mm) under the pressure of 50 bar with the addition of binder (rubber glue, dissolved in hexane). The obtained pellets were sintered at 1200 °C for 24 h in air. The densities of the sintered samples were determined by the Archimedes method. The values were ~ 80–85% of the theoretical density. After polishing, the opposite surfaces of the pellets were painted with the silver-palladium paste (Ag70-Pd30 weight %) and sintered at 900 °C in air.
The electrical properties of the samples were studied by impedance spectroscopy using an Elins Z-1000P impedance meter. The impedance spectra were measured by the 2-probe method over the frequency range of 100–106 Hz in the temperature interval of 300–900 °C with the step of 20 °C. The cooling rate was 1 °C/min. The exposure time at each temperature was 1 h. The sample resistances were determined using the ZView software.
The conductivity was measured in dry air, humid air, dry nitrogen, and humid nitrogen. Air and nitrogen were dried by passing gas through calcium chloride, zeolites, and phosphorus pentoxide P2O5 (pH2O = 3.5·10–5 bar). The humidified atmosphere was obtained by bubbling gas through Drexel flasks with a 30% (by weight) aqueous solution of sodium hydroxide NaOH and a saturated solution of potassium bromide KBr (pH2O = 2·10–2 bar). The humidity inside the experimental setup was monitored by the HIH-4000 gas humidity sensor.
Electrical conductivity measurements were also performed at different oxygen partial pressures (pO2). The impedance spectra were measured in the temperature range of 400 − 900 °C with pO2 ranging between 10–19 and 0.21 bar. The electrochemical oxygen pump and sensor based on Y-stabilized ZrO2 were used for controlling and monitoring pO2 near the samples. All the electrochemical data were recorded under equilibrium conditions.
The electrical conductivity was calculated by the well-known equation:
where R is resistance of a sample, l is its thickness, and S is the cross-sectional area.
Results and discussion
X-ray diffraction analysis
Figure 1 shows the Rietveld refined XRPD pattern of BaLa2In2O7. The XRPD analysis revealed that the undoped compound BaLa2In2O7 was single phase. It can be characterized by the tetragonal symmetry (sp. gr. P42/mnm). The refined unit cell parameters for BaLa2In2O7 (Table 1) are in good agreement with reported data [21, 25].
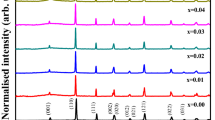
The XRPD analysis showed that the BaCaxLa2-xIn2O7-0.5× samples with x = 0.05, 0.10 were single phase. Their structure can be characterized by the same space group as for the undoped composition. For x = 0.15, 0.20, 0.25, 0.30, 0.4, and 0.5, all obtained samples contained impurities even after the additional heat treatment at 1200 °C. The XRPD patterns revealed the presence of the very small quantities of BaCa2In6O12 (x = 0.15) and CaO (x ≥ 0.25) impurities indicating that the solubility limit of calcium is within the range of 0 ≤ x ≤ 0.10 at 1100–1200 °C. Thus, the homogeneity region of the BaCaxLa2-xIn2O7-0.5× solid solution is limited by the BaCa0.1La1.9In2O6.95 composition. As an example, Figs. 2 and 3 show the Rietveld refined XRPD patterns for the samples with x = 0.05, 0.30. The unit cell parameters and R-factors are listed in Table 1. The do** with calcium within the homogeneity region of the BaCaxLa2-xIn2O7-0.5× solid solution leads to a slight decrease in the a parameter and an increase in the c parameter, and as a result to a reduction in the unit cell volume.
The obtained results indicate that the homogeneity region of Ca2+ − substituted solid solution is relatively small. Comparison of the homogeneity regions for BaMxLa2–xIn2O7–0.5x (M = Sr2+, Ba2+) solid solutions [23] shows that it increases in the row Ca2+ (0 ≤ x ≤ 0.10) − Sr2+ (0 ≤ x ≤ 0.20) − Ba2+ (0 ≤ x ≤ 0.30). From a crystallographic point of view, calcium is closer in size to lanthanum, but the introduction of ions with larger ionic radius is more preferable for this structure (\({r}_{{\text{L}}{\text{a}}^{3+}}\)=1.216 Å, \({r}_{{\text{C}}{\text{a}}^{2+}}\)=1.18 Å, \({r}_{{\text{S}}{\text{r}}^{2+}}\)=1.31 Å, \({r}_{{\text{B}}{\text{a}}^{2+}}\)=1.47 Å for CN 9 [26]) as it leads to an expansion of the rock-salt-type layers. It should be noted that such trend is different from that observed for the perovskite structure of LaMO3. For example, the most favorable energies and hence higher solubilities are predicted for Ca/Sr in LaMO3 [27]. Among alkaline earth dopants for LaMO3, the solution energies were the highest for Mg2+ and Ba2+. However, for lanthanum located in the rock-salt layer in the RP structure, this trend is not preserved and substitution of La3+ with larger cations is more preferable.
Transport properties
The electrical conductivity was determined by the impedance spectroscopy in the temperature range of 300–900 °C. Figure 4a–d shows typical impedance spectra for the BaCa0.05La1.95In2O6.95 and BaLa2In2O7 samples. As can be seen, no significant differences were observed in the impedance spectra of the doped and parent samples. A comparison of the impedance spectra measured in air and in nitrogen is shown in Fig. 4b. As can be seen, in the main region of the studied frequencies, one semicircle is observed. The semicircle is characterized by small capacitances ~ 10–11–10–12 F. Hence, it can be related to the bulk properties of the sample. The values of bulk resistance were obtained and the conductivity was calculated by Eq. (1).
To determine the nature of the predominant conductivity and the stability of the obtained phases at low oxygen partial pressures, total conductivity was studied while varying pO2. Figure 5a shows the pO2 dependence of electrical conductivity at different temperatures in dry atmosphere for the sample with x = 0.05 as an example. As it can be seen, the electrical conductivity values increase with increasing pO2 in the range of 10–3 − 0.21 bar. This behavior indicates the presence of hole conduction. The oxygen vacancies, generated due to do** or from Frenkel defects, can react with oxygen producing holes:
As the RP phases show the hole contribution to the total conductivity even in the absence of the acceptor-type dopant (oxidizing conditions), the following alternative quasi-chemical reaction for the formation of holes can be written:
In both cases, the hole concentration increases with increasing pO2.
The total conductivity does not depend on pO2 in a wide interval of 10–19 − 10–3 bar. This indicates that the oxygen-ion transport is predominant in these conditions as the oxygen vacancy concentration is fixed by dopant content. This typical ionic conductivity plateau should be attributed to the disordering in the oxygen sublattice (in other words to the intrinsic and extrinsic oxygen vacancies in the structure). The hole conductivity in accordance with the quasi-chemical Eqs. (2) and (3) is expected to pO21/4 dependence. Thus, in air, the total conductivity can be represented by the following expression:
where K is the hole conductivity at pO2 = 1 bar. The total conductivity at high temperatures (T > 600 °C) and oxidizing condition (pO2 > 10–3 bar) is proportional to pO21/4, indicating mixed (O2− and h•) nature of conductivity. With the decrease in temperature the slope becomes flatter indicating that the hole contribution decreases and the oxygen-ion contribution increases. Fitting of Eq. (4) to the experimental data allows us to separate the total conductivity into ionic and electronic contributions. Partial contributions of conductivities will be discussed below.
It should be noted that the dotted line in Fig. 5a shows the partial pressure of oxygen in nitrogen atmosphere. As can be seen, it corresponds to the plateau region where ionic conductivity is predominant. This is important when discussing the data on conductivity measured in nitrogen.
The total conductivity as a function of pO2 was also measured in wet atmosphere (Fig. 6a). Small positive slopes can be observed for isotherms at 710 and 614 °C (oxidizing conditions). Isotherms at 519 and 425 °C are almost independent of pO2 in the whole pO2 range studied, which indicates the ionic nature of the conductivity. The presence of the proton contribution leads to an increase in the ionic conductivity, and under oxidizing conditions, the hole contribution decreases.
The electrical conductivity versus pO2 for the Ca2+ − doped sample with x = 0.10 exhibits similar dependece compared to that for the sample with x = 0.05. The obtained data demonstrate that the samples are chemically stable at low pO2 as there are no deviations from linear behavior.
Using the obtained data which are shown in Figs. 5a and 6a, the ion transport numbers as tion = σion / σtot (σion is the conductivity value corresponding to the plateau) were calculated (Figs. 5b and 6b). It was found that the investigated phases are a pure ionic conductors with tion ≈ 1 at pO2 < 10–4 bar, and they are mixed conductors above 10–4 bar (dry atmosphere). In air (pO2 = 0.21 atm) for Ca2+ − doped samples, the ionic transport numbers (Fig. 5b increase with decreasing temperature (23% at 900 °C and 80% at 425 °C) and below 500 °C, the phases exhibit predominant oxygen-ion transport. The tion decreases with increasing temperature in air probably due to the difference in the activation energies between the ionic and the electronic conduction. At the same time, for the undoped phase, it was previously shown [25] that under dry air in a wide temperature range, the compound demonstrated mixed ionic-electronic (hole) conductivity and the oxygen-ion transfer numbers did not exceed 20%. That is, Ca2+ − do** led to a significant increase in the share of ion transport.
For wet conditions in air, the samples exhibit predominant ionic conductivity below 600 °C, due to appearance of proton contribution. It should be noted that the undoped phase exhibited dominant proton transport only at lower temperatures, below 300–350 °C [25]. That is, do** made it possible to expand the temperature range of predominance of proton transport.
In this way, the studied phases exhibit a mixed type of conductivity in dry air at high temperatures; the ionic transport numbers increase due to proton contribution in wet air; the measurement of conductivity in nitrogen allows us to determine the ionic conductivity.
The temperature dependences of the conductivity for the studied BaCaxLa2-xIn2O7-0.5x (x = 0, 0.05, 0.10, 0.15) samples in dry air and in nitrogen are shown in Fig. 7. It should be noted that the sample with the concentration of dopant x = 0.15 is out of the homogeneity range (0 ≤ x ≤ 0.10) determined earlier and its conductivity can be affected by the impurities. The dependence of conductivity versus dopant concentration will be discussed later. The comparison of conductivities measured in different atmospheres is presented for the sample x = 0.05 as an example (Fig. 8).
Some trends can be identified:
-
the conductivity measured in nitrogen is lower than that measured in air (Fig. 8); the increase in conductivity in air can be explained by the contribution of electronic conductivity due to the formation of electron holes in oxidizing conditions, as shown by the quasi-chemical reactions (2) and (3);
-
as the temperature decreases, the differences between different atmospheres decrease, indicating an increase in the contribution of ionic transport;
-
do** led to the conductivity increase demonstrating the role of oxygen vacancies in the O2− transport.
The values of electrical conductivity obtained in nitrogen are indicated on the dependences of the electrical conductivity versus pO2 (Fig. 5a). As can be seen, they are within the plateau region, so we can conclude that the conductivities measured in nitrogen are ionic conductivities.
The temperature dependences of the conductivity in wet atmosphere are shown in Fig. 9 and the comparison of conductivities in different atmospheres is shown for the sample BaCa0.05La1.95In2O6.975 in Fig. 8. In nitrogen atmosphere, the conductivity increases with increasing humidity, which is due to the appearance of proton conduction. The process of dissociative intercalation of water and the appearance of proton defects can be represented by the following quasi-chemical reaction:
where \({V}_{O}^{\bullet\bullet}\) is oxygen vacancy, \({O}_{o}^{\mathrm{x}}\) is the oxygen atom in a regular position, and \(O{H}_{o}^{\bullet}\) is the hydroxyl group in the oxygen sublattice.
Moreover, for materials with the block structure, there is an alternative quasi-chemical reaction showing the possible insertion of protons into the rock-salt-type blocks:
where \({(\text{OH)}}_{\text{O}}^{\bullet}\) is the hydroxyl group in the oxygen position, \(\mathrm{(OH)_i^\prime}\) is the hydroxyl group in the interstitial site of the rock-salt-type block:
The effect of humidity for air atmosphere is due to the appearance of proton defects and a decrease in the concentration of holes:
This is accompanied by the decrease in the hole conductivity and the increase in proton conductivity. Therefore, there is no increase in conductivity in wet air relative to dry air in comparison with the “dry nitrogen-wet nitrogen” changes. The conductivities in wet nitrogen and wet air are comparable at low temperatures.
Figure 10 demonstrates the dependences of the total (in air) and ionic (in nitrogen) conductivities versus dopant concentration. As can be seen within the homogeneity region for BaCaxLa2-xIn2O7-0.5× with the increase in x, the conductivity increases. This behavior can be explained by an increase in the number of oxygen vacancies (dry conditions) and the appearance of proton conduction (wet conditions) as a result of acceptor do**. The BaCa0.15La1.85In2O6.925 sample showed the lowest conductivity due to the presence of impurities.
The activation energies of conductivity were calculated using the obtained data with the equation σT = σo·exp(–Ea/kT) (σo is the pre–exponential factor, Ea is the activation energy, k is the Boltzmann constant and T is the absolute temperature). The activation energies are given in Table 2. As can be seen, the activation energy increases with increasing x in dry nitrogen (i.e. the activation energy for oxygen-ion conductivity). This is not a favorable factor for the oxygen-ion transport. The effect of higher activation energy is suppressed by the increase in concentration of oxygen vacancies, as a result, the oxygen-ion conductivity increases. The increase in activation energy is probably due to the appearance of shorter Ca-O bond length (and stronger) compared to La-O.
It is of interest to compare the oxygen-ion transport of isostructural phases BaMxLa2–xIn2O7–0.5x (M = Sr2+, Ba2+) [23] to understand the effect of the size factor on the ionic transport. Figure 11 compares the oxygen-ion conductivity dependences versus the concentration of dopant for these solid solutions. Within the homogeneity regions (for the same concentration of oxygen vacancies at x = 0.10), the ionic conductivity increases in the row Ca2+ − Ba2+ − Sr2+; this trend does not correlate with the increase in the unit cell volume for Ca2+ − Sr2+ − Ba2+. As it is known, an increase in the lattice parameter (and cell volume) contributes to an increase in the mobility of an ion, as a result of a decrease in the average metal-oxygen bond energy [28]. On the other hand, another parameter correlated with anion transport is the crystallographic free volume (defined as the difference between the unit cell volume and the volume of all atoms). Figure 12 shows the dependences of the unit cell volume and the free volume for the BaMxLa2–xIn2O7–0.5x (M = Ca2+, Sr2+, Ba2+) solid solutions. As can be seen, the combination of two factors leads to the fact that strontium is the optimal dopant for facilitated oxygen migration.
The conductivity isotherms for the BaCaxLa2–xIn2O7–0.5× solid solution in wet atmosphere exhibit the similar trends (Fig. 13). The do** also leads to an increase in conductivity when the proton contribution of conductivity appears. This behavior reflects the fundamental relationship between oxygen-ion and proton conductivity, because proton transport occurs between the adjacent oxygen sites and the «softening» of the proton environment leads to the increase in mobility of protonic charge carriers [29].
The proton conductivity (\({\sigma }_{{H}^{+}}\)) was calculated by subtracting the oxygen-ion conductivity (\({\sigma }_{{O}^{2-}}\)) measured in dry N2 (plateau in Fig. 5a is \({\sigma }_{{O}^{2-}}\)) from ion conductivity obtained in wet N2 atmosphere (plateau in Fig. 6a is \({\sigma }_{{H}^{+}}+{\sigma }_{{O}^{2-}}\)): \({\sigma }_{{H}^{+}}={\sigma }^{\mathrm{wet}}-{\sigma }^{\mathrm{dry}}\). The proton conductivities for the studied solid solutions are compared in Fig. 14. The dependencies reflect the same trend as the ones obtained in wet nitrogen. Do** leads to the increase in proton conductivity, which is due to the appearance of oxygen vacancies. Within the narrow homogeneity region of BaCaxLa2-xIn2O7-0.5x, the increase in proton conductivity is small. For the solid solution with Ba, a monotonic increase in the proton conductivity occurs within the entire homogeneity range. For the Sr2+ − solid solution, with the intermediate width of the homogeneity range, the maximum of the proton conductivity is reached at x = 0.15.
That is, as the concentration of the dopants continues to increase, the mobility of protons decreases, which is usually described as the effect of their “trap**” by an acceptor impurity that has a negative charge (for example, the formation of such complexes of acceptor-bound defects \((\mathrm{M_{La}^\prime}\cdot\mathrm{OH_o^\bullet})^\times\)). These effects are described in detail for doped ABO3 perovskites [30]. At relatively high-dopant concentrations, the contribution of “free” protons decreases. This process is accompanied by an increase in the activation energy due to additional contribution of the “trap**” energies ΔEH. As shown, indeed, the activation energy of proton conduction increases with increasing x (Table 2).
The nature of dopant can also affect the proton “trap**” [30]. Obviously, with a decrease in size of the alkaline earth metal cation, this effect increases; for the Ba2+-containing phases, the effect of proton “trap**” was less pronounced. Therefore, more significant increase in conductivity is observed for BaLa2–xBaxIn2O7–0.5x. Higher proton conductivities are reached at a higher Ba2+ concentration.
Comparison of the electrical properties of the studied phases with conventional doped one-layered RP compound BaLaInO4 and the LaInO3 perovskite shows that the acceptor-type do** improves the conductivity in the row Ca, Sr, Ba for BaLaInO4 [9], but for the La0.9Me0.1InO3-δ (Me = Mg, Ca, Sr, Ba) indates conductivity increases in the row Mg, Ca, Ba, Sr [31]. The proton conductivities for one-layered BaLaInO4 and Ca2+, Sr2+, and Ba2+-doped phases are also shown in Fig. 14 [16]. The proton conductivities of undoped and Sr2+-doped phases are lower than those for double-layered RP phases (the difference is especially significant for undoped phases); the Ca2+ do** is less advantageous for the double-layered RP phases compared to the Sr2+ and Ba2+ do**.
Thus, the double-layered RP phases combine the properties of both layered structures and 3D-structures, and in general, it can be concluded that the increase in the number of perovskite blocks in the series of (AO)(ABO3)n phases increases their role in ion transport.
Conclusions
The homogeneity range of the BaCaxLa2–xIn2O7–0.5× solid solution exists in the composition region of 0 ≤ x ≤ 0.10. The electrical conductivity of BaCaxLa2–xIn2O7–0.5× was measured as a function of temperature and oxygen partial pressure in dry and wet atmosphere. The ionic conductivity contributions (oxygen-ion conductivity and proton conductivity) in BaCaxLa2-xIn2O7-0.5× were determined. The Ca2+ − do** led to an increase in conductivity by 0.25 orders of magnitude (500 °C), in addition, the share of oxygen-ion transport increased with decreasing temperature (23% at 900 °C and 80% at 425 °C) and was higher than the ion transport numbers for the undoped phase (20% in the same temperature range). In wet air, the ionic transport dominates below 600 °C due to the appearance of the proton contribution. The do** led to an expansion of the temperature range of predominance of proton transport.
The comparison of conductivity data for the BaMxLa2-xIn2O7-0.5x (where M = Ca, Sr, Ba) solid solutions showed that the suitable dopant to the La sites of BaMxLa2-xIn2O7-0.5× should have larger ionic radius than the host cation to improve the ionic conductivity. At the same time, for the correlation of ion transport and geometrical parameters, the correlation of the unit cell volume and the free volume should be taken into account.
References
Troncoso L, Alonso JA, Fernández-Díaz MT, Aguadero A (2015) Introduction of interstitial oxygen atoms in the layered perovskite LaSrIn1+xBxO4+δ system (B = Zr, Ti). Solid State Ion. https://doi.org/10.1016/j.ssi.2015.09.014
Troncoso L, Alonso JA, Aguadero A (2015) Low activation energies for interstitial oxygen conduction in the layered perovskites La1+xSr1-xInO4+δ. J Mater Chem A. https://doi.org/10.1039/C5TA03185K
Matsuhira T, Kurahashi Y, Hasegawa K, Ihara M (2018) Proton conducting properties of Sr1+XLn1-XAlO4-δ (Ln=Pr,Sm) with layered perovskite structure for solid oxide fuel cells, 232nd ECS Meeting 2017, National Harbor, Maryland, USA, Vol. MA2017–0, Curran Ass. Inc. Red Hook, NY, 2018 2, I02-Ionic and Mixed Conducting Ceramics 11 (IMCC 11). https://doi.org/10.1149/MA2017-02/39/1710
Li C, Dammak H, Dezanneau G (2019) Identification of oxygen diffusion mechanisms in Nd1-xAExBaInO4-x/2 (AE=Ca, Sr, Ba) compounds through molecular dynamics. Phys Chem Chem Phys. https://doi.org/10.1039/C9CP03048D
Lee D, Lee HN (2017) Controlling oxygen mobility in Ruddlesden-Popper oxides. Materials. https://doi.org/10.3390/ma10040368
Troncoso L, Mariño C, Arce MD, Alonso JA (2019) Dual oxygen defects in layered La1.2Sr0.8-xBaxInO4+δ (x = 0.2, 0.3) oxide-ion conductors: a neutron diffraction study. Materials. https://doi.org/10.3390/ma12101624
Shiraiwa M, Kido T, Fujii K, Yashima M (2021) High-temperature proton conductors based on the (110) layered perovskite BaNdScO4. J Mater Chem A. https://doi.org/10.1039/D0TA11573H
Tarasova N, Animitsa I, Galisheva A (2020). J Solid state electrochem. https://doi.org/10.1007/s10008-020-04630-1
Tarasova N, Animitsa I (2022) Materials AIILnInO4 with Ruddlesden-Popper structure for electrochemical applications: relationship between ion (oxygen-ion, proton) conductivity, water uptake, and structural changes. Materials. https://doi.org/10.3390/ma15010114
Fujii K, Esaki Y, Omoto K, Yashima M, Hoshikawa A, Ishigaki T, Hester JR (2014). Chem Mater. https://doi.org/10.1021/cm500776x
Shiraiwa M, Fujii K, Esaki Y, Kim SJ, Lee S, Yashima MJ (2017) Crystal structure and oxide-ion conductivity of Ba1+xNd1-xInO4-x/2. J Electrochem Soc. https://doi.org/10.1149/2.0411713jes
Tarasova N, Galisheva A, Animitsa I, Anokhina I, Gilev A, Cheremisina P (2022) Novel mid-temperature Y3+ → In3+ doped proton conductors based on the layered perovskite BaLaInO4. Ceram Int. https://doi.org/10.1016/j.ceramint.2022.02.102
Tarasova N, Galisheva A, Animitsa I, Korona D, Davletbaev K (2022) Novel proton-conducting layered perovskite based on BaLaInO4 with two different cations in B-sublattice: synthesis, hydration, ionic (O2-, H+) conductivity. Int J Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2022.04.112
Tarasova N, Galisheva A, Animitsa I (2021) Ba2+/Ti4+-co-doped layered perovskite BaLaInO4: the structure and ionic (O2−, H+) conductivity. Int J Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2021.02.044
Tyitov YuO, Byilyavina NM, Markyiv VYa, Slobodyanik MS, Krajevs’ka YaA (2009) Synthesis and crystal structure of BaLaInO4 and SrLnInO4 (Ln–La, Pr). Reports of National Academy of Science of Ukraine 10:160–166 (in Ukrainian)
Tarasova N, Animitsa I, Galisheva A, Korona D (2019) Incorporation and conduction of protons in Ca, Sr, Ba-doped BaLaInO4 with Ruddlesden-Popper structure. Materials. https://doi.org/10.3390/ma12101668
Caldes M, Michel C, Rouillon T, Hervieu M, Raveau B (2002) Novel indates Ln2BaIn2O7, n= 2 members of the Ruddlesden–Popper family (Ln= La, Nd). J Mater Chem. https://doi.org/10.1039/b108987k
Tyitov YuO, Byilyavina NM, Markyiv VYa, Slobodyanik MS, Krajevs’ka YaA, Yaschuk VP (2010) Synthesis and crystal structure of BaLn2In2O7, Reports of National Academy of Science of. Ukraine 1:148–154 (in Ukrainian)
Tarasova N, Bedarkova A, Animitsa I, Abakumova E (2023) Cation and oxyanion do** of layered perovskite BaNd2In2O7: oxygen-ion and proton transport. Int J Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2022.11.172
Tarasova N, Bedarkova A, Animitsa I, Abakumova E, Gnatyuk V, Zvonareva I (2022) Novel protonic conductor SrLa2Sc2O7 with layered structure for electrochemical devices. Materials. https://doi.org/10.3390/ma15248867
Tarasova N, Galisheva A, Animitsa I, Korona D, Abakumova E, Medvedev D (2022) Novel mixed oxygen-electronic conductors based on BaLa2In2O7 with two-layer Ruddlesden-Popper structure. Ceram Int. https://doi.org/10.1016/j.ceramint.2022.08.139
Tarasova N, Bedarkova A, Animitsa I, Abakumova E, Belova K, Kreimesh H (2022) Novel high conductive ceramic materials based on two-layer perovskite BaLa2In2O7. Int J Mol Sci. https://doi.org/10.3390/ijms232112813
Tarasova N, Bedarkova A, Animitsa I, Belova K, Abakumova E, Cheremisina P, Medvedev D (2022) Oxygen ion and proton transport in alkali-earth doped layered perovskites based on BaLa2In2O7. Inorganics. https://doi.org/10.3390/inorganics10100161
Tarasova N, Bedarkova A, Animitsa I, Verinkina E (2022) Synthesis, hydration processes and ionic conductivity of novel gadolinium-doped ceramic materials based on layered perovskite BaLa2In2O7 for electrochemical purposes. Processes. https://doi.org/10.3390/pr10122536
Tarasova N, Galisheva A, Animitsa I, Korona D, Kreimesh H, Fedorova I (2022) Protonic transport in layered perovskites BaLanInnO3n+1 (n = 1, 2) with Ruddlesden-Popper structure. Appl Sci. https://doi.org/10.3390/app12084082
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr, Sect A: Found Crystallogr. https://doi.org/10.1107/S0567739476001551
Islam MS, Cherry M (1997) Protons in LaMO3: atomistic modelling and ab initio studies. Solid State Ionics. https://doi.org/10.1016/S0167-2738(97)00067-2
Sammells AF, Cook RL, White JH, Osborne JJ, MacDuff RC (1992) Rational selection of advanced solid electrolytes for intermediate temperature fuel cells. Solid State Ionics. https://doi.org/10.1016/0167-2738(92)90097-9
Kreuer KD (1999) Aspects of the formation and mobility of protonic charge carriers and the stability of perovskite-type oxides. Solid State Ionics. https://doi.org/10.1016/S0167-2738(99)00188-5
Putilov LP, Tsidilkovski VI (2019) Impact of bound ionic defects on hydration of acceptor-doped proton-conducting perovskites. Phys Chem Chem Phys. https://doi.org/10.1039/C8CP07745B
He H, Huang X, Chen L (2000) Sr-doped LaInO3 and its possible application in a single layer SOFC. Solid State Ionics. https://doi.org/10.1016/S0167-2738(00)00666-4
Funding
This work received research funding from the Ministry of Science and Higher Education of the Russian Federation (Ural Federal University Program of Development within the Priority-2030 Program) is gratefully acknowledged. The equipment of the Ural Center for Shared Use “Modern nanotechnology” Ural Federal University (Reg.№ 2968) was used with the financial support of the Ministry of Science and Higher Education of the Russian Federation (Project № 075–15-2021–677).
Author information
Authors and Affiliations
Contributions
Irina Animitsa: conceptualization, data curation, writing—review and editing, supervision, funding acquisition, project administration; Daniil Korona: investigation, formal analysis, writing—original draft, resources; Artem Gilev: writing—review and editing, visualization, investigation; Abdurahmon Mubinov: investigation, formal analysis, writing—original draft; Hala Kreimesh: investigation, formal analysis, writing—original draft; Natalia Tarasova: validation, resources, funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Animitsa, I., Korona, D., Gilev, A. et al. Synthesis and electrical properties of BaCaxLa2-xIn2O7–0.5× solid solution with double-layered Ruddlesden-Popper structure. J Solid State Electrochem 28, 1849–1860 (2024). https://doi.org/10.1007/s10008-023-05710-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-023-05710-8