Abstract
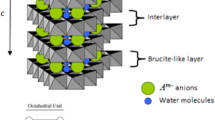
The modification of lutetium hydroxide on a layered double hydroxide (LDH), [Ni4Al(OH)10]NO3, is carried out by coprecipitation and thereafter hydrothermal treatment. When the content of Lu is less, only X-ray diffractions (XRD) due to [Ni4Al(OH)10]NO3 are found. When the content is 2.89 wt% or more in the product, a series of low-intensity diffractions is identified, which can be indexed with a triclinic cell, space group \( P\overline{1} \). After the modification, the a lattice parameter for Ni-Al layered double hydroxide does not show any considerable changes, which means Lu3+ could not be incorporated into M(OH)2 (M=Ni, Al) layers because of its larger ion radius (85 pm). According to the observations by scanning electron microscopy (SEM) or transmission electron microscopy (TEM), with low Lu content, single nanorods appear among the disks of [Ni4Al(OH)10]NO3 and turn into bundles with high Lu content. The modification of Lu prevents the structural transformation of the Ni-Al LDH into β-Ni(OH)2. It can be found that the transformation is stopped by 0.9 wt% Lu when being soaked in 7.0 mol L−1 potassium hydroxide (KOH) for 72 days at 60 °C, or by 1.29 wt% Lu after the 25th charging/discharging cycle at 60 °C in the same alkali solution. The modification also improves the high-temperature performances of the electrode. At a high temperature of 60 °C, the 2.89 wt% Lu-modified [Ni4Al(OH)10]NO3 has a more slowly reduced capacity of 265.8 mAh g−1 after 25 charge/discharge cycles under a current density of 800 mA g-1 in 7.0 mol L−1 KOH, while the [Ni4Al(OH)10]NO3 has a sharply reduced capacity of 202.7 mAh g−1 under the same condition.














Similar content being viewed by others
References
Hu WK, Noreus D (2003) Chem Mater 15:974–978
Hu M, Gao X, Lei L, Sun Y (2009) J Phys Chem C 113:7448–7455
Gao X-P, Yang H-X (2010) Energ Environ Sci 3:174–189
Cheng FY, Liang J, Tao ZL, Chen J (2011) Adv Mater 23:1695–1715
Li J, Shangguan E, Guo D, Tian M, Wang Y, Li Q, Chang Z, Yuan X-Z, Wang H (2014) J Power Sources 270:121–130
Oshitani M, Takayama T, Takashima K, Tsuji S (1986) J Appl Electrochem 16:403–412
Caravaggio GA, Detellier C, Wronski Z (2001) J Mater Chem 11:912–921
Zhao YL, Wang JM, Chen H, Pan T, Zhang JQ, Cao CN (2004) Int J Hydrogen Energy 29:889–896
Hu WK, Gao XP, Noreus D, Burchardt T, Nakstad NK (2006) J Power Sources 160:704–710
Rives V (2001) Layered double hydroxides: present and future. Nova Science Publishers Inc., New York
Khan AI, Ragavan A, Fong B, Markland C, O’Brien M, Dunbar TG, Williams GR, O’Hare D (2009) Ind Eng Chem Res 48:10196
Guo X, Zhang F, Evans DG, Duan X (2010) Chem Commun 46:5197
Hu M, Lei LX (2007) J Solid State Electrochem 11:847–852
Lei L, Hu M, Gao X, Sun Y (2008) Electrochim Acta 54:671–676
Yang Z, Hu M, Ji X, Lei L (2011) Chin J Appl Chem 28:1323–1330
Hu M, Ji X, Lei L, Lu X (2013) J Alloys Compd 578:17–25
Hu M, Ren F, Lei L, Lu X (2013) Sep Purif Technol 120:198–205
Hu M, Yang Z, Lei L, Sun Y (2011) J Power Sources 196:1569–1577
Hu M, Ji X, Lei L, Lu X (2013) Electrochim Acta 105:261–274
Hu M, Lei L, Chen J, Sun Y (2011) Electrochim Acta 56:2862–2869
Qin L, Hu M, Gao X, Lei L (2011) J Solid State Electrochem 15:405–412
Oshitani M, Watada M, Shodai K, Kodama M (2001) J Electrochem Soc 148:A67–A73
Tanaka T, Kuzuhara M, Watada M, Oshitani M (2006) J Alloys Compd 408:323–326
Fang Q, Cheng Y, Jian X, Zhu L, Yu H, Wang Z, Jiang L (2010) J Rare Earths 28:72–78
Gupta V, Kusahara T, Toyama H, Gupta S, Miura N (2007) Electrochem Commun 9:2315–2319
Gándara F, Perles J, Snejko N, Iglesias M, Gómez-Lor B, Gutiérrez-Puebla E, Monge MÁ (2006) Angew Chem Int Ed 45:7998–8001
Geng F, Matsushita Y, Ma R, **n H, Tanaka M, Iyi N, Sasaki T (2009) Inorg Chem 48:6724–6730
Žák Z, Unfried P, Giester G (1994) J Alloys Compd 205:235–242
Giester G, Žák Z, Unfried P (2009) J Alloys Compd 481:116–128
Zhang J, Liu Z, Lin J, Fang J (2005) Cryst Growth Des 5:1527–1530
Jia G, Zheng Y, Liu K, Song Y, You H, Zhang H (2008) J Phys Chem C 113:153–158
Vucelic M, Moggridge GD, Jones W (1995) J Phys Chem 99:8328–8337
Aicken AM, Bell IS, Coveney PV, Jones W (1997) Adv Mater 9:496–500
Ren X, Sun B, Tsai C-C, Tu Y, Leng S, Li K, Kang Z, Horn RMV, Li X, Zhu M, Wesdemiotis C, Zhang W-B, Cheng SZD (2010) J Phys Chem B 114:4802–4810
Gao X, Lei L, Hu M, Qin L, Sun Y (2009) J Power Sources 191:662–668
Espinós JP, González-Elipe AR, Odriozola JA (1987) Appl Surf Sci 29:40–48
Ren J, Yan J, Zhou Z, Wang X, Gao X (2006) Int J Hydrogen Energy 31:71–76
Chigane M, Ishikawa M (1998) J Chem Soc Faraday Trans 94:3665–3670
Cotton FA, Wilkinson G (1988) Advanced inorganic chemistry. Wiley-Interscience, New York
Wang X, Li Y (2003) Chem Eur J 9:5627–5635
Wang L, Li B, Zhao X, Chen C, Cao J (2012) PLoS ONE 7:e37781
Mi X, Gao XP, Jiang CY, Geng MM, Yan J, Wan CR (2004) Electrochim Acta 49:3361–3366
Li WY, Zhang SY, Chen J (2005) J Phys Chem B 109:14025–14032
Fan J, Yang Y, Yang Y, Shao H (2007) Electrochim Acta 53:1979–1986
He XM, Jiang CY, Li W, Wan CR (2006) J Electrochem Soc 153:A566–A569
Bard AJ, Faulkner LR (2003) Electrochemical methods: fundamentals and applications. Wiley, New York
Liu B, Wang XY, Yuan HT, Zhang YS, Song DY, Zhou ZX (1999) J Appl Electrochem 29:855–860
Motupally S, Streinz CC, Weidner JW (1998) J Electrochem Soc 145:29–34
Zhu W-H, Ke J-J, Yu H-M, Zhang D-J (1995) J Power Sources 56:75
Zhang X, Gong Z, Zhao S, Geng M, Wang Y, Northwood DO (2008) J Power Sources 175:630–634
Li J, Shangguan E, Guo D, Li Q, Chang Z, Yuan X-Z, Wang H (2014) J Power Sources 263:110–117
Acknowledgments
This project is supported by the Science Research Foundation of Shanghai Institute of Technology, Grant No. YJ2014-20, China Postdoctoral Science Foundation, Grant No. 2011M500840, Jiangsu Planned Projects for Postdoctoral Research Foundation, Grant No. 1101009C, and Postdoctoral Science Fund of Southeast University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, M., Zuo, S., Yang, R. et al. Modification of lutetium hydroxide for the structural and electrochemical stability of Ni-Al layered double hydroxide. J Solid State Electrochem 19, 671–683 (2015). https://doi.org/10.1007/s10008-014-2651-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-014-2651-4