Abstract
Objective
The purpose of this study was to test the hypothesis that general anesthesia (GA) plus thoracic epidural anesthesia (TEA) has no impact on the outcomes of off-pump coronary artery bypass surgery (OPCABs) compared to GA followed by patient-controlled TEA (PCTEA), while GA plus TEA leads to a higher requirement for vasoactive drug use.
Methods
Sixty-four patients, American Society of Anesthesiologists physical status II and III, who were scheduled for elective OPCABs, were offered an epidural catheter inserted at the T2–3 interspace and then randomized into 1 of 2 groups according to whether TEA was applied intraoperatively. The TEAperio group received GA plus TEA, while the TEApost group received GA alone. All groups had postoperative PCTEA. The number of requirements for vasoactive drugs and the extubation times were recorded. The analgesic effect was monitored by visual analog scale (VAS) pain scores. Heart rate, blood pressure, and blood gases were also monitored. The data are presented as mean values ± standard deviation, or medians with quartiles.
Results
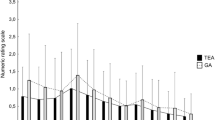
The proportion of vasoactive drug use was significantly higher in the TEAperio group intraoperatively (before or during completion of anastomoses: 59.4 vs. 20.7%, p = 0.004; after completion of anastomoses: 53.1 vs. 17.2%, p = 0.007). There was no statistically significant difference in extubation times or VAS scores between the 2 groups.
Conclusions
We conclude that GA plus TEA has no impact on the outcomes of OPCABs, while its use leads to a higher requirement for vasoactive drug use. GA followed by PCTEA facilitates the anesthesia administration, while it does not affect the extubation time and the postoperative analgesic effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Combined thoracic epidural anesthesia (TEA) and general anesthesia (GA) followed by postoperative patient-controlled TEA (PCTEA) has been extensively investigated during off-pump coronary artery bypass surgery (OPCABs) because of its potential beneficial effects, although, because of the difficulties in performing an adequately sized, randomized, controlled trial, evidence is still lacking that TEA could reduce the main outcomes of OPCABs [1]. TEA has been shown to have favorable effects on pulmonary function parameters [2, 3], to improve the quality of analgesia, [4], and to have beneficial effects in cardiac risk patients [4, 5].
On the other hand, the epidural administration of local anesthetics during TEA leads to sympatholysis; the hemodynamic effects of which combine with those of the GA, thereby increasing the risk of severe intraoperative hypotension [6], which may offset the positive hemodynamic effects of TEA. A study [7] showed that during OPCABs, the requirement for vasoactive drug use in the combined TEA and GA group was about three times than that in the GA-alone group. Hogan [6] also found that approximately 44% of TEA patients required vasopressors to maintain mean arterial blood pressure (MAP) above 70 mmHg during OPCABs. A high frequency of vasoactive drug use may increase the difficulty of intraoperative anesthesia administration.
We designed a prospective, randomized study in OPCABs patients to test the hypothesis that GA plus TEA has no impact on the outcomes of OPCABs, while it leads to a higher requirement for vasoactive drug use.
Subjects, materials, and methods
Institutional review board approval and patients’ written informed consents were obtained for the study. From September 2006 to October 2008, 62 patients (American Society of Anesthesiologists [ASA] physical status II and III) scheduled to have elective OPCABs and who met the following criteria were enrolled: stable angina with a documented stenosis diameter of at least 50% in 2 or more epicardial coronary arteries, left ventricular ejection fraction >30%, no history of cerebrovascular disease, and normal coagulation profile (prothrombin time >80% and partial thromboplastin time in the normal range, platelet counts >100000/ml). Antiplatelet medications were withdrawn at least a week before surgery in all patients in the test group and the control group (see below for group details). Heparin infusion, if present, was discontinued 6 h before performing epidural catheterization. Low-molecular-weight heparin was stopped 12 h before epidural catheterization.
β-Adrenergic blockers were continued during the perioperative period, but angiotensin-converting enzyme inhibitors, calcium channel blockers, and other cardiovascular medications were stopped the day before surgery. Premedication consisted of oral lorazepam, 2 mg, and morphine, 0.1 mg/kg intramuscularly, 90 min preoperatively; intravenous (IV) cefazoline, 30 mg/kg, was injected before instrumentation. Monitoring included arterial and central venous blood pressure, electrocardiogram with continuous ST-segment analysis (leads II and V5), pulse oximeter, and end-tidal carbon dioxide. A new ST depression of ≤0.1 mV or new ST elevations of ≥0.2 mV lasting more than 1 min were the criteria for the judgment of intra- and postoperative ischemia. The software we used supported the examination of the tape recordings via computerized ST-segment analysis. With the patient in the lateral position on the operating room table, an epidural catheter was inserted 3–5 cm into the epidural space at the T1–T2 or T2–T3 interspace. A standard test dose (80 mg of 2% plain lidocaine) excluded intrathecal or vessel placement of the epidural catheter.
Patients were randomly assigned to one of two groups according to whether intraoperative thoracic epidural analgesia was applied. The TEAperio group received GA plus TEA, while the control group (TEApost group) received GA alone. TEA was induced by the epidural administration of 0.1 ml/kg of a mixture of 0.1% bupivacaine and 2 μg/ml fentanyl, as a loading dose to achieve a sensory level up to dermatome T1. TEA was maintained by a continuous epidural infusion of the same mixture (0.1 ml/kg/h). The TEApost group received the equivalent volume flow rate of saline. The research team members completed epidural puncture or tested the sensory level. Neither the patient nor the anesthesiologist was aware of the study assignment; furthermore, the epidural drug was labeled with only the patients’ name and the infusion instructions.
The induction of GA was achieved in all patients with propofol, 1–2 mg/kg; fentanyl, 2–3 μg/kg; and vecuronium, 0.1 mg/kg, to facilitate tracheal intubation. The lungs were ventilated in normocapnia with an air-oxygen mixture. GA was maintained by infusions of propofol and sufentanil to a target anesthesia depth of 40–50 BIS, using a bispectral index monitor (BIS A2000 Monitor; Aspect Medical System, Newton, MA, USA) in both groups. The total intraoperative doses of propofol and sufentanil were recorded. Additional boluses of vecuronium were injected if necessary.
After internal mammary artery harvesting, 100 IU/kg of bovine lung heparin was given and anticoagulation was assessed every 30 min by the celite-activated coagulation time, with the trigger level for additional heparin set at 300 s in OPCABs. The heparin dose was administered after 60 min had elapsed from epidural puncture. On completion of the distal and proximal coronary anastomoses, heparin was antagonized with protamine sulfate at a 1:1 ratio (1 mg/kg). All OPCAB surgeries were performed via a midline sternotomy; mechanical stability of the coronary arteriotomy area was achieved with a suction stabilizer, and a soft plastic coronary flow shunt was always introduced into the coronary arteriotomy. A heating mattress was used, and infusion of warm fluids was performed to maintain normothermia. The use of vasoconstrictors or vasodilators during surgery followed institutional protocols. Hypotension was treated by anesthesia-depth modulation and ephedrine or norepinephrine boluses, or both, if necessary, and hypertension was treated by anesthesia-depth modulation and nicardipine or nitroglycerin, at the discretion of the attending anesthesiologist.
At the end of the procedure, all groups had postoperative PCTEA and then were transferred intubated to the intensive care unit (ICU) without reversal of muscle relaxant drugs. PCTEA, with a standard PCA pump (CADD-PCA; Deltec, St. Paul, MN, USA), was achieved by epidural bolus doses of 2 ml of the mixture of 0.1% bupivacaine plus 2 μg/ml fentanyl, a lockout interval of 20 min, and a background epidural infusion of 0.1 ml/kg/h. Low-molecular-weight heparin (LMWH) 5000 U was applied subcutaneously once a day. The first LMWH dose was administered 6–8 h postoperatively. Epidural infusion was generally maintained until the third postoperative day. The catheters were removed on the surgical ward by an anesthesiologist after checking the coagulation status and the scheduled antiplatelet or anticoagulant therapy.
Weaning from the ventilator was started when the following criteria were achieved: hemodynamic stability, no significant arrhythmias, no major bleeding, temperature >36°C, adequate level of consciousness and no signs of neurologic injury, adequate pain control, pH and blood gases within normal values with an FIO2 of <60%, and a positive end-expiratory pressure of <6 cmH2O. The patients were eligible for transfer from the ICU when the following criteria were met: SpO2 >90% at an FIO2 of ≤50% by a facemask, adequate cardiovascular stability with no hemodynamically significant arrhythmias, chest tube drainage <50 ml/h, urine output >0.5 ml/kg, no intravenous inotropic or vasopressor therapy, and no seizures. Criteria for hospital discharge were as follows: stable hemodynamics without arrhythmias, clean and dry incisions, normal body temperature, normal bowel movement, and independent ambulation and feeding.
During the whole process of OPCABs and a postoperative period of 48 h, the number of myocardial ischemia events for every patient was recorded. The following observations were recorded 1 h after operation (T0), 10 min after extubation (T1), and every 2 h for the next 12 h (T2, T4, T6, T8, T10, T12): heart rate (HR), MAP, arterial oxygen tension (PaO2), and arterial carbon dioxide tension (PaCO2). Every 2 h after extubation the patients were assessed for pain, by visual analog scale (VAS) scores at rest and while coughing, on a scale of 10 (0, no pain; 10, maximum pain). Adequate postoperative analgesia was defined as a VAS score of ≤3. For both groups, a VAS score of >3 was managed by the following algorithm: (1) The patient could use PCTEA; (2) if analgesia remained inadequate, IV ketorolac, 30–60 mg, could be dosed at the discretion of the attending physician; and (3) if analgesia still remained inadequate, epidural fentanyl could be administered by a physician as a 50–100 μg bolus. The number of requirements for PCTEA, ketorolac, fentanyl, and vasoactive drugs was recorded, as well as the extubation time.
According to our preliminary experiments, the proportion of vasoactive drug use in the TEAperio group was approximately 70%, a reduction in the proportion of 30% was considered clinically significant. To detect this reduction at a 5% level of significance with 95% power, 30 patients were required in each group. Therefore, a total of 64 patients were included in our study. Descriptive statistics are summarized as means ± standard deviation, whereas categoric variables are expressed in percentages. Baseline and outcome variables were compared using the Student unpaired t-test and Pearson χ2 test where appropriate. The effects on the VAS score were evaluated by a two-way analysis of variance for repeated measurements. All analysis was done with SPSS 13 for Windows (SPSS, Chicago, IL, USA). A p value of ≤0.05 was considered significant.
Results
Of the 64 patients, 61 completed the study. One patient from the TEAperio group was withdrawn from the study because of failure to catheterize the epidural space. Two patients were excluded because of a bloody tap during insertion of the epidural catheter, and surgery was delayed for 24 h. There was no in-hospital mortality in either group. Each group had similar demographic characteristics (Table 1).
Operative time, number of distal anastomoses, and number of Maze procedures were similar in the TEAperio and TEApost group patients, as were the perioperative features of myocardial ischemia, ICU stay, and ventilation time (Table 2). The TEAperio group did not differ from the TEApost group in intraoperative medications except for requiring less sufentanil and propofol, and being more inclined to use vasoactive drugs. The proportion of vasoactive drug use in the TEA group was significantly higher than that in no-TEA group during surgery, whereas the proportion was similar in the two groups postoperatively (Table 2).
There were no differences between the two groups with respect to postoperative pain control at rest and on coughing, and there were also no differences between the two groups in postoperative respiratory and hemodynamic variables (p > 0.05) (Table 3).
Discussion
To our knowledge, this was the first prospective randomized trial of the anesthesia-analgesia regimen of GA followed by PCTEA in patients undergoing OPCABs. The major finding of the present study was that this anesthesia–analgesia regimen, compared to GA plus TEA followed by PCTEA, facilitated anesthesia administration by reducing the proportion of vasoactive drug use, while the regimen had no effect on postoperative ventilation time, pain control, or hemodynamic and respiratory variables.
TEA has been used for coronary artery surgery since 1989 at a few centers [8]. Though randomized studies [9] of TEA have been unable to report effects on main outcomes in patients undergoing OPCABs, probably because of insufficient statistical power, TEA has recently gained popularity because of its potential beneficial effects on the perioperative stress response, analgesia, and postoperative pulmonary function. Studies have shown that TEA permitted earlier extubation, [10] decreased pain scores, [11], and had the potential to decrease the incidence of lower respiratory tract infections, acute renal failure, and acute confusion [12] compared with conventional treatment with intravenous opioids.
The use of TEA in patients who receive perioperative anticoagulation has been questioned because of the theoretical increased risk of epidural hematoma formation. Currently, insufficient data and experience are available to determine whether the risk of epidural hematoma is increased when combining TEA with the heparin anticoagulation of cardiac surgery. The second American Society of Regional Anesthesia and Pain Medicine (ASRA) Consensus Conference advised that unfractionated heparin administration should be delayed for 1 h after needle placement [13]. It has been suggested that epidural catheter removal is potentially traumatic, and that the coagulation status should be normalized before catheter removal [14]. In the present study, LMWH was used routinely, so the catheter was removed a minimum of 10–12 h after the last dose of LMWH. Subsequent LMWH dosing was administered a minimum of 2 h after catheter removal. In addition, the postoperative monitoring of neurologic function and the selection of neuraxial solutions that minimize sensory and motor block are recommended to facilitate the detection of epidural hematoma formation.
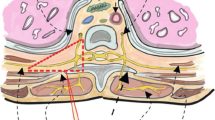
TEA has the potential for blocking cardiac afferent and efferent fibers, which originate from the first through fifth thoracic levels (T1 through T5). Ideally, in patients undergoing OPCABs, TEA should provide hemodynamic stability by dilating constricted coronary vessels, decreasing the heart rate (HR) and myocardial metabolism, reducing pre- and afterload, and optimizing oxygen availability [15, 16]. However, TEA has an inherent risk of cardiovascular depression and arterial vasodilation [17]. The hemodynamic effects of combined TEA and GA increase the risk of severe intraoperative hypotension [6], which may offset the positive hemodynamic effects of TEA. It is certainly reasonable to support low blood pressure with a vasopressor (such as phenylephrine), as is frequently used with TEA techniques. Would hemodynamic instability, particularly the occurrence of hypotension in the TEA group, increase the difficulty of intraoperative anesthesia administration? In the present study the intraoperative proportion of vasoactive drug use in the TEAperio group was much higher than that in the TEApost group (before or during completion of anastomoses: 59.4 vs. 20.7%, respectively; p = 0.004; after completion of anastomoses: 53.1 vs. 17.2%, respectively; p = 0.007). We believed that combined TEA and GA would significantly increase the probability of hemodynamic instability, particularly the occurrence of hypotension, and thus increase the difficulty of intraoperative administration of anesthesia, though there was no difference in myocardial ischemia events between the two groups.
Our study showed that the TEAperio group consumed less sufentanil and propofol than the TEApost group. However, the mechanism whereby TEA reduced propofol consumption is not yet clear. It is controversial whether epidural anesthesia has an effect on BIS during GA. Many studies have shown that epidural anesthesia had no effect on the BIS, and suggested that the BIS may not be a good indicator when GA is combined with epidural anesthesia [18], while other investigators have documented that epidural anesthesia decreased the BIS and reduced the requirements for propofol or volatile anesthetics during GA [19, 20]. Furthermore, some studies have shown that the quality of the epidural blockade may, consequently, affect general anesthetic requirements [21, 22]. The present study suggested that TEA may synergistically decrease BIS in GA patients, thus reducing the amount of propofol consumption.
Combined GA and TEA enables more rapid extubation of patients after OPCABs compared with conventional anesthetic management [23]. One study has shown that maximal inspiratory lung volumes are approximately 30% larger (250–300 ml tidal volume increase) in patients receiving TEA than in those receiving GA, and this increase leads to significantly quicker extubation times [24]. The time to extubation is shortened in patients receiving TEA, probably as a result of several factors, which include lower intraoperative requirements for sufentanil and propofol, postoperative avoidance of parenteral opioids, analgesia, and in particular, better body temperature control at the end of surgery [25]. In the present study, there was no difference in the duration of mechanical ventilation between the TEAperio and TEApost groups (p = 0.237), which indicated that postoperative epidural analgesia was responsible for early extubation, while the lower requirement of sufentanil and propofol was not a main factor. The reason for the lack of importance of the sufentanil and propofol requirement in this context may be that extubation time is generally 4 h after surgery, when sufentanil and propofol may have been almost completely metabolized.
In the present study, PCTEA was achieved by the epidural infusion of a mixture of bupivacaine plus fentanyl, while IV ketorolac or epidural fentanyl could be administered if analgesia was inadequate. There was no difference in VAS scores or in the number of requirements for ketorolac or fentanyl between the TEAperio and TEApost groups within 12 h after extubation. This finding indicated that, after extubation, the analgesic effect was mainly derived from the epidural analgesia, while the effects of the intraoperative analgesics had been almost completely lost.
Recently, a prospective, randomized, controlled trial [26] has shown that the addition of TEA to conventional GA accounts for a significant reduction in the incidence of postoperative arrhythmias, and an improvement in the overall quality of recovery, allowing earlier tracheal extubation and earlier hospital discharge. In our study, GA plus TEA had no impact on the outcomes of OPCABs, while it lead to a higher requirement for vasoactive drug use. Perioperative myocardial infarction events, ventilation time, and ICU stay were similar in the TEAperio and TEApost group patients. Our results were not in contradiction with those of the trial noted above [26]. We think GA plus TEA can improve the outcomes of OPCABs, not because of the reduction of anesthesia and analgesic drugs during OPCABs, but because of the excellent analgesia that TEA provided postoperatively.
The results of the present study suggest that GA followed by PCTEA is a feasible anesthesia–analgesia regimen in patients undergoing OPCABs. This regimen guarantees earlier extubation time and excellent postoperative analgesia, while it reduces the probability of the need for vasoactive drugs and thus facilitates intraoperative anesthesia administration.
References
Ishibe Y, Shiokawa Y, Umeda T, Uno H, Nakamuram M, Izumi T. The effect of thoracic epidural anesthesia on hypoxic pulmonary vasoconstriction in dogs: an analysis of the pressure-flow curve. Anesth Analg. 1996;82:1049–55.
Hachenberg T, Holst D, Ebel C, Pfeiffer B, Thomas H, Wendt M, Hedenstierna G. Effect of thoracic epidural anaesthesia on ventilation–perfusion distribution and intrathoracic blood volume before and after induction of general anaesthesia. Acta Anaesthesiol Scand. 1997;41:1142–8.
Wulf H. Combination of thoracic epidural anesthesia and general anesthesia. Luxury or value for money? Anaesthesist. 1999;48:357–8.
Blomberg S, Emanuelsson H, Kvist H, Lamm C, Ponten J, Waagstein F, Ricksten SE. Effects of thoracic epidural anesthesia on coronary arteries and arterioles in patients with coronary artery disease. Anesthesiology. 1990;73:840–7.
Meissner A, Rolf N, Aken HV. Thoracic epidural anesthesia and the patient with heart disease: benefits, risks, and controversies. Anesth Analg. 1997;85:517–28.
Hogan Q. Cardiovascular response to sympathetic block by regional anesthesia. Reg Anesth. 1997;21:26–34.
Salvi L, Sisillo E, Brambillasca C, Juliano G, Salis S, Marino MR. High thoracic epidural anesthesia for off-pump coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2004;18:256–62.
Joachimsson PO, Nystrom SO, Tyden H. Early extubation after coronary artery surgery in efficiently rewarmed patients: a postoperative comparison of opioid anesthesia versus inhalational anesthesia and thoracic epidural analgesia. J Cardiothorac Vasc Anesth. 1989;3:444–54.
Scott NB, Turfrey DJ, Ray DA, Nzewi O, Sutcliffe NP, Lal AB, Norrie J, Nagels WGB, Ramayya GP. A prospective randomized study of the potential benefits of thoracic epidural anesthesia and analgesia in patients undergoing coronary artery bypass grafting. Anesth Analg. 2001;93:528–35.
Priestley MC, Cope L, Halliwell R, Gibson P, Chard RB, Skinner M, Klineberg PL. Thoracic epidural anesthesia for cardiac surgery: The effects on tracheal intubation time and length of hospital stay. Anesth Analg. 2002;94:275–82.
Royse C, Royse A, Soeding P, Blake D, Pang J. Prospective randomized trial of high thoracic epidural analgesia for coronary artery bypass surgery. Ann Thorac Surg. 2003;75:93–100.
Moore CM, Cross MH, Desborough JP, Burrin JM, Macdonald IA, Hall GM. Hormonal effects of thoracic extradural analgesia for cardiac surgery. Br J Anaesth. 1995;75:387–93.
Horlocker TT, Wedel DJ, Benzon H, Brown DL, Enneking FK, Heit JA, Mulroy MF, Rosenquist RW, Rowlingson J, Tryba M, Yuan CS. Regional anesthesia in the anticoagulated patient: defining the risks (the second ASRA consensus conference on neuraxial anesthesia and anticoagulation). Reg Anesth. 2003;28:172–97.
Wulf H. Epidural anaesthesia and spinal haematoma. Can J Anaesth. 1996;43:1260–71.
Blomberg S, Emanuelsson H, Ricksten SE. Thoracic epidural anesthesia and central hemodynamics in patients with unstable angina pectoris. Anesth Analg. 1989;69:558–62.
Saada M, Catoire P, Bonnet F, Delaunay L, Gormezano G, Macquin-Mavier I, Brun P. Effect of thoracic epidural anesthesia combined with general anesthesia on segmental wall motion assessed by transesophageal echocardiography. Anesth Analg. 1992;75:329–35.
Rolf N, Weber TP, Aken HV. Hypotension during thoracic surgery under combined general and high thoracic epidural anesthesia. Tech Reg Anesth Pain Manag. 2000;4:161–6.
Pollock GE, Neal JM, Liu SS. Sedation during spinal anesthesia. Anesthesiology. 2000;93:728–34.
Ishiyama T, Kashimoto S, Oguchi T, Yamaguchi T, Okuyama K, Kumazawa T. Epidural ropivacaine anesthesia decreases the bispectral index during the awake phase and sevoflurane general anesthesia. Anesth Analg. 2005;100:728–32.
Hodgson PS, Liu SS, Gras TW. Does epidural anesthesia have general anesthetic effects? A prospective, randomized, double-blind, placebo-controlled trial. Anesthesiology. 1999;91:1687–92.
Liu SS, Ware Rajendran S. Effects of concentration and volume of 2-chloroprocaine on epidural anesthesia in volunteers. Anesthesiology. 1997;86:1288–93.
Sakura S, Sumi M, Kushizaki H, Saito Y, Kosaka Y. Concentration of lidocaine affects intensity of sensory block during lumbar epidural anesthesia. Anesth Analg. 1999;88:123–7.
Salvi L, Parolari A, Veqlia F, Brambillasca C, Grequ S, Sisillo E. High thoracic epidural anesthesia in coronary artery bypass surgery: a propensity-matched study. J Cardiothorac Vasc Anesth. 2007;21:810–5.
Crescenzi G, Landoni G, Monaco F, Bignami E, De Luca M, Frau G, Rosica C, Zangrillo A. Epidural anesthesia in elderly patients undergoing coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2009;23:807–12.
Dhole S, Mehta Y, Saxena H. Comparison of continuous thoracic epidural and paravertebral blocks for postoperative analgesia after minimally invasive direct coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2001;15:288–92.
Caputo M, Alwair H, Rogers CA, Pike K, Cohen A, Monk C, Tomkins S, Ryder I, Moscariello C, Lucchetti V, Angelini GD. Thoracic epidural anesthesia improves early outcomes in patients undergoing off-pump coronary artery bypass surgery: a prospective, randomized, controlled trial. Anesthesiology. 2011;114:380–90.
Acknowledgments
The authors thank the thoracic surgeons and the general surgeons for allowing their patients to be studied; and Dr. Nan Ma for statistical advice.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Liang, Y., Chu, H., Zhen, H. et al. A prospective randomized study of intraoperative thoracic epidural analgesia in off-pump coronary artery bypass surgery. J Anesth 26, 393–399 (2012). https://doi.org/10.1007/s00540-012-1325-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-012-1325-6