Abstract
Background
Light-to-moderate intensity strength training (LMST) improves muscular strength, physical functioning, and some side effects in head and neck cancer survivors (HNCS). Heavy lifting strength training (HLST) may further improve these outcomes; however, it has not been studied in HNCS. The primary aim of the LIFTING trial was to examine the feasibility and safety of a HLST program in HNCS ≥1-year post-surgical neck dissection.
Methods
In this single-arm feasibility study, HNCS were asked to complete a twice weekly, 12-week, supervised HLST program, gradually progressing to lifting heavy loads of 80–90% of 1 repetition maximum (1RM) for barbell squat, bench press, and deadlift. The feasibility outcomes included recruitment rate, 1RM completion rate, program adherence, barriers, and motivation. The preliminary efficacy outcomes included changes in upper and lower body strength.
Results
Nine HNCS were recruited over an 8-month period during the COVID-19 pandemic. All 9 (100%) completed the 1RM tests and successfully progressed to heavy loads at approximately 5 weeks. The median attendance was 95.8% (range 71–100%), and few barriers were reported. Weight lifted increased for squat/leg press (median change: +34kg; 95% CI +25 to +47), bench press (median change: +6kg; 95% CI +2 to +10), and deadlift (median change: +12kg; 95% CI +7 to +24). No adverse events were reported and participants were motivated to continue HLST after the study.
Conclusions
HLST appears feasible and safe for HNCS and may result in meaningful improvements in muscular strength. Future research should consider additional recruitment strategies and compare HLST to LMST in this understudied survivor population.
Trial registration
NCT04554667
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The standard treatment for early-stage head and neck cancer (HNC) is surgery or radiotherapy. Multiple modalities, mainly chemoradiotherapy, are used for locally advanced HNC [1]. Despite improvements in treatments, HNC survivors (HNCS) still experience acute and chronic side effects [1,2,3,4,5,6,7] including dental and oral complications, nutritional deficits, speech and voice impairments, immune suppression, infectious complications, shoulder dysfunction, pain, shortness of breath, weakness, physical fatigue, difficulty slee**, poor appetite, self-consciousness, low self-esteem, and reduced quality of life (QoL) [1, 3, 7,8,9]. Interventions to improve shoulder function, muscular strength, and physical functioning are needed to facilitate return to work and improve overall QoL.
Strength training improves some of these side effects in HNCS [10,11,12,13,14,15,16]; however, most studies to date have tested light-to-moderate intensity strength training (LMST) rather than heavy load intensities [8, 10,11,12,13,14,15, 17,18,19]. LMST involves lifting lighter loads more times (i.e., 10–15 repetitions) whereas heavy lifting strength training (HLST) involves lifting heavier loads fewer times (i.e., 1–6 repetitions) [20]. While LMST interventions have been shown to be beneficial in HNCS, research in other populations has shown that training with heavy loads may maximize strength gains [20, 21]. These findings raise the possibility that a HLST program in HNCS may have even greater benefits for muscular strength, symptom management, and QoL. To date, however, no study has examined a HLST program in any HNCS groups.
The primary purpose of the Heavy Lifting Strength Training in Head and Neck Cancer Survivors (LIFTING) study was to test the feasibility and safety of a HLST program in HNCS at least 1-year post-surgical neck dissection (ND). A secondary purpose was to examine the preliminary efficacy of HLST for improving muscular strength, physical functioning, and patient-reported outcomes (PROs). We hypothesized that HNCS who have undergone a surgical ND would be willing and able to participate safely in a HLST program. We also hypothesized that there would be meaningful increases in muscular strength and patient-reported outcomes from baseline to post-intervention.
Materials and methods
Setting and participants
This study was conducted at the University of Alberta in Edmonton, Alberta, Canada, and received ethics approval from the Health Research Ethics Board of Alberta Cancer Committee (HREBA-CC). HNCS were recruited from (a) a HNC surgical clinic at the University of Alberta Hospital, (b) the Cancer Rehabilitation Clinic in the Faculty of Rehabilitation Medicine at the University of Alberta, (c) the Head and Neck Cancer Support Society, and (d) self-referral.
Eligibility criteria included (1) any subtype and stage of HNC including thyroid cancer; (2) at least 1-year post-ND with full shoulder range of motion or recovery of the spinal accessory nerve (SAN); (3) aged 18 and older; (4) no unmanaged medical conditions, alcohol, or drug abuse; (5) approved for a HLST program by the treating surgeon and a certified exercise physiologist; and (6) ability to communicate in English. The participants were excluded if they were currently involved in an exercise trial or a clinical drug trial.
Design and procedures
This single-arm study involved assessments before and after the 12-week HLST intervention. Potentially eligible HNCS were identified and screened for eligibility by a nurse and physiotherapist during follow-up visits and rehabilitation sessions. HNCS were put in touch with the study coordinator via email and/or phone for more information about the study. The participants were asked to complete online questionnaires using the Research Electronic Data Capture (REDCap) and scheduled for in-person physical assessments at the Behavioural Medicine Fitness Centre at the University of Alberta. Final eligibility was determined through objective measurements of shoulder flexion and abduction range of motion. The participants were required to meet or exceed the following age-based cut points for shoulder range of motion: participants 18–50 years old: ≥150° for flexion and abduction; participants over 50 years old: ≥130° for flexion and abduction.
Exercise intervention
Participants took part in a 12-week, supervised, HLST exercise program 2 days per week, while progressively working towards lifting loads of 80–90% of 1RM for squat, bench press, and deadlift. Exercise prescription intensities were calculated from the 1RM scores at baseline. Participants were guided through a 5–10 min warmup and cooldown consisting of static stretching. For the HLST intervention, exercises consisted of 3 main movements (squat, bench press, and deadlift) as well as accessory/auxiliary movements (face pulls, pushups, dumbbell lunges, farmers carry, and planks). There was an individualized 5- to 8-week progression/adaptation period to heavy loads (80–90% of 1RM). Typical intensity progressions are presented in Fig. S1. For safety purposes, measurements of heart rate and blood pressure were taken before each session, and fatigue and pain were assessed before and after each session.
The HLST exercise intervention included bench press, but no overhead pressing movements. Overhead exercises have been associated with higher levels of trapezius muscle activity in patients with SAN injury following ND surgery and may not be feasible in this group due to regional pain resulting from subacromial im**ement and glenohumeral joint restriction [12]. Each main movement was performed for 1–6 repetitions (80–90%) of 1RM, and an RPE of 8–9 on the 10-point Borg CR-10 Scale for squat, bench press, and deadlift, for a total of 3–5 sets. Once participants progressed to heavy loads, rest periods of 3–5 min between sets were required.
The intensity of the 3 primary movements was based on 1RM and RPE. To progress the intensity, it has been recommended that a 2–10% increase (lower percent for small muscle mass exercises, higher percent increase for large muscle mass exercises) in load be applied when the individual can perform the current workload for one to two repetitions over the desired number for two consecutive training sessions [22]. Total volume (sets × reps × load) was increased if heavy loads were tolerated well (i.e., participants reported >1 RPE less than the predicted RPE for two consecutive sessions). Once participants reached heavy loads (80% 1RM), the total volume was gradually progressed at this intensity by increasing sets and reps within our defined parameters (3–5 sets of 1–6 reps), before increasing intensity to 85% and then 90% 1RM. Accessory movements were also included in the training protocol in addition to the 3 main movements as they target smaller muscle groups in comparison to multi-joint, compound exercises which target multiple larger, muscle groups.
Demographic, behavioral, and medical characteristics
Demographic and behavioral variables were assessed using self-report. Baseline exercise was assessed with the Godin Leisure Time Exercise Questionnaire (GLTEQ) [23]. Medical data was extracted from medical records.
Feasibility and safety outcomes
Feasibility was assessed by the eligibility rate, recruitment rate, adherence rate, follow-up assessment rate, perceived benefits, perceived barriers, and motivation. Perceived benefits, barriers, and motivation for the HLST program were assessed at post-intervention (within 1 week of completing the HLST program) using standard items based on the Theory of Planned Behavior [24]. Safety was assessed by patient-reported and observed adverse events during exercise testing and supervision.
Health-related fitness outcomes
Health-related fitness outcomes were assessed at baseline (within 1 week prior to starting the HLST program) and at post-intervention (within 1 week of completing the HLST program). 1RM strength tests were performed to determine the maximum amount of weight each participant could lift one time for each of the main exercises: barbell squat, bench press, and deadlift. The 1RM strength testing protocol is available online as Supplementary Material 1.
Body composition was assessed using measurements of hip-to-waist ratio, height, and weight. Additional functional fitness assessments for participants 50 years and up included the 6-min walk test (6MWT) and 30-s sit to stand. Shoulder flexion and abduction range of motion were measured in all participants using a goniometer to ensure ranges met the cut points required for study eligibility outlined above.
Patient-reported outcomes
Patient-reported outcomes were assessed at baseline (within 1 week of starting the HLST program) and at post-intervention (within 1 week of completing the HLST program) by questionnaires completed on REDCap. QoL was measured using the European Organization for the Research and Treatment of Cancer QoL Questionnaire-C30 (EORTC QLQ-C30) [25]. Post-traumatic growth was assessed using the Post Traumatic Growth Inventory (PTGI) [26]. Fear of cancer recurrence was measured using the Fear of Cancer Recurrence Inventory (FCRI) [27]. Cancer-specific QoL was measured using the Functional Assessment of Cancer Therapy-Head and Neck (FACT-H&N Symptom Index) [28]. Anxiety was measured using the Spielberger State Trait Anxiety Inventory (STAI) [29]. Fatigue was assessed using the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) questionnaire [30]. Perceived stress was measured using the Perceived Stress Scale (PSS) [31]. Impairment as a result of the cancer treatment was measured using the Neck Dissection Impairment Index (NDII) [32]. Symptom burden was assessed using the revised Edmonton Symptom Assessment System (ESAS-r) [33]. Self-esteem was measured using the Rosenberg Self-Esteem Scale (RSE) [34]. Typical sleep habits were measured using the Insomnia Severity Index (ISI) [35].
Statistical analysis
For this single-arm feasibility and safety study, our goal was to recruit 15–20 HNCS over a 6–month period. Descriptive analyses were used to report the eligibility rate, recruitment rate, adherence rate, assessment rate, adverse event rate, benefits, barriers, and motivation. Changes from baseline to post-intervention in muscular strength and patient-reported outcomes were reported as median change with 95% confidence interval.
Results
Eligibility rate, recruitment rate, and baseline characteristics
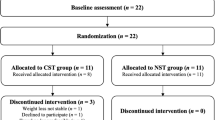
The flow of participants through the LIFTING trial is presented in Fig. 1. From November 2020 to June 2021 (8 months), 16 potentially eligible and interested HNCS were referred by clinic staff to the study coordinator for further review. Due to the COVID-19 pandemic, staff redeployment, and suspension of in-person recruitment, we were unable to track how many HNCS in the two clinics were screened, deemed ineligible, approached, or declined. In total, 9 HNCS were recruited to the study. The baseline characteristics of the 9 participants are reported in Table 1.
Feasibility of maximal strength testing and supervised HLST
All 9 participants (100%) successfully completed the baseline maximal strength testing, although some issues and challenges were experienced and are summarized in Table S6. All 9 participants (100%) who completed the baseline maximal strength testing attempted the HLST program. Of the 9 participants who began the HLST program, the median attendance was 23 (95.8%) of the 24 supervised exercise sessions with a range of 17 (71%) to 24 (100%). Details pertaining to the feasibility of the HLST exercise program are summarized in Table S7. The most common reasons for missed or delayed exercise sessions were household chore/shoveling-related back strains, out of town for vacation or work, visiting family, medical procedure, and death in the family. The mean duration of each exercise session was 60 min, and all 9 participants (100%) progressed to heavy loads (80–90% of 1RM) at session #9 or #10 (approximately 5 weeks). Two participants (22.2%) substituted the barbell squat with the leg press machine due to lower body instability or an inability to execute the exercise safely. Sets and reps were decreased for 1 participant’s bench press due to feeling fatigued that day. All 9 participants (100%) reached a peak volume of 5 sets and 6 repetitions at 90% 1RM in at least one of the main exercises (squat, bench press, and/or deadlift). For 1 participant (11.1%), we exceeded 90% of their predicted squat 1RM for 4 sessions, as they did not perform a true 1RM test at baseline. All 9 participants (100%) responded well to an increase in total volume (sets × reps × load) from session to session after heavy loads were reached. No exercise-related adverse events were observed or reported during testing or the supervised HLST sessions.
Follow-up assessment rate
Seven participants (77.7%) completed all follow-up maximal strength tests. One participant was cautious on all follow-up strength tests as they did not want to aggravate previous back muscle pain. Another participant could not complete the deadlift at light loads (50% baseline 1RM) due to extreme stomach pain the previous night and day of testing. Eight participants (88.8%) completed the follow-up questionnaires in their entirety. One participant (11.1%) declined to answer questions about engaging in a HLST program over the next 6 months because she said she could not predict the future.
Benefits, barriers, and motivation
Descriptive statistics for perceived benefits of HLST are presented in Table S8. All 9 participants (100%) believed that the HLST program was highly beneficial for their physical fitness and muscular strength. Eight participants (88.8%) reported better sense of control over health and better overall quality of life after the HLST program. Seven participants (77.7%) reported improved levels of fatigue to carry out daily activities. Two participants (22.2%) reported worse shoulder/neck pain or injury and one participant (11.1%) reported worse fatigue and worse fear of cancer recurrence. Descriptive statistics for perceived barriers to HLST are presented in Table 2. No barrier severely interfered with participation in the HLST program. Descriptive statistics for changes in motivational and behavioral outcomes are presented in Table S9. Motivation was high as baseline and remained high post-intervention, with little to no changes. Descriptive statistics for participants’ motivation to continue HLST after the LIFTING trial are presented in Table 3. Five of the 8 participants (62.5%) felt very motivated, and 3 participants (37.5%) felt somewhat motivated to continue HLST over the next 6 months.
Effects of HLST on health-related fitness and patient-reported outcomes
Descriptive statistics for changes in health-related fitness outcomes are presented in Table 4. Meaningful improvements were evident for all strength exercises, blood pressure, and resting heart rate. The percentage changes in maximal strength of the 3 strength exercises and combined weight lifted are presented in Fig. 2. Back squat strength improved by 48%, bench press by 22%, deadlift by 12%, and total load lifted increased by 34% from baseline to post-intervention. Minimal changes were reported in secondary physical functioning outcomes including active shoulder ranges of motion (flexion and abduction), the 6MWT, the 30-s sit-to-stand test, weight, and waist-to-hip ratio.
Descriptive statistics for changes in health-related QoL are presented in Table S10. Minimal changes were reported in functional or symptom scales; however, there was a meaningful improvement for the global health status/QoL measure (median change: +8.3; 95% CI +4.2 to +17.0). Descriptive statistics for changes in patient-reported symptoms, and psychosocial outcomes are presented in Table S11. Minimal changes were reported.
Discussion
Overall, preliminary findings of the LIFTING trial suggest that a HLST program may be feasible and safe for HNCS at least 1-year post-ND. Due to the suspension of in-person recruitment during the COVID-19 pandemic, however, we were unable to recruit our desired sample size of 15–20 HNCS over a 6-month period or track the recruitment and eligibility rates in our study. Of the 16 potentially eligible and interested HNCS referred to the study coordinator, 9 HNCS (56.3%) were eligible, interested, and completed the HLST program. The main reasons for ineligibility in our study were not meeting the shoulder flexion and abduction requirements, and uncontrolled comorbid disease. In the future, HNC trials should consider recruiting from multiple cancer centers or via a cancer registry.
All 9 participants completed all muscular strength tests; however, modifications to the 1RM test were made for 2 participants. One participant was unwilling to continue to increase the weight to find their 1RM for the squat and stopped at a peak 3RM. Another participant was unwilling to continue finding their 1RM for the deadlift and stopped at a true 2RM. Neither of these participants stopped due to pain, injury, or discomfort. Their predicted 1RM was calculated based on these values. As a whole, 1RM testing was feasible in the LIFTING trial.
The median attendance to the HLST program was 95.8%, and few modifications had to be made to the HLST prescription. The majority of study participants reported benefits in their physical fitness and muscular strength, better sense of control over health, better quality of life, improved levels of fatigue to carry out daily activities, better ability to stop thinking about their cancer, better shoulder/neck pain or injury, and better shoulder/neck motion as a result of HLST. No barrier severely interfered with their ability to participate in HLST. Overall, behavioral and motivational outcomes were high at baseline and remained high post-intervention. The majority of study participants perceived HLST to be beneficial and enjoyable and were motivated to continue HLST after the LIFTING trial.
Our study suggested meaningful improvements in upper and lower body muscular strength (squat/leg press, bench press, and deadlift) from a total combined weight of 170 to 227 kg, a 34% improvement. The 1RM strength improvements for squat/leg press, bench press, and deadlift were 48%, 22%, and 12%, respectively. Although there is no literature that involved free weight HLST in HNCS, one randomized controlled trial in newly diagnosed breast cancer patients scheduled for adjuvant therapy showed that a 12-week leg press program consisting of 4 sets of 4 repetitions at 90% 1RM resulted in improved lower body strength by 20kg (20%) and also improved walking economy and functional performance compared to the control group [36].
Furthermore, exercise studies in HNCS involving LMST have reported changes in muscular strength post-intervention. A prospective, randomized controlled trial in HNCS examined an upper body, 12-week progressive resistance exercise training program consisting of 2 sets of 10–15 repetitions at 60–70% 1RM. The authors reported improvements in upper body strength of 16.3 kg (37%) for the seated row and 7.1 kg (24%) for chest press in the progressive resistance exercise training group, which were superior to results from the standard therapeutic group [10]. In a prospective pilot study, oropharyngeal cancer patients participated in a 12-week progressive resistance exercise training program during chemoradiotherapy consisting of 2–3 sets of 15 repetitions, working to 8 repetitions at heavier loads; intensity percentage was not specified. The results demonstrated decreases in 1RM leg press strength of −6 kg (−4%) and chest press strength of −7 kg (−12%) possibly due to participant medical complications, but the dip in strength experienced during treatment was mostly regained during follow-up [17].
Despite improvements in maximal strength, our study showed minimal changes in secondary health-related fitness outcomes including active shoulder ranges of motion, the 6MWT, the 30-s sit-to-stand test, weight, and waist-to-hip ratio. The LIFTING trial was designed to maximize strength gains rather than target flexibility or cardiovascular fitness. It is possible that our study participants’ performance on the 6MWT or sit-to-stand tests was not limited by their muscular strength given their high level of performance. The study was also designed to increase muscle mass; however, these changes may not have been captured by measures of body weight or waist-to-hip ratio. Future studies should assess body composition directly using measures such as dual-energy X-ray absorptiometry imaging.
Our study also suggested a meaningful improvement in global health status/QoL; however, there were minimal changes in all other functional or symptom scores. These minimal changes may be because participants reported excellent QoL at baseline, and levels remained high post-HLST. In addition, one participant had extreme stomach pain the night before and day of post-intervention testing, which may have influenced their self-reported data. Minimal changes were also reported in patient-reported symptoms and psychosocial outcomes from baseline to post-HLST. One systematic review demonstrated that exercise was safe and feasible and may lead to improvements in muscular strength, lean body mass, physical function, fatigue management, and QoL in HNC patients [16]. Overall, our data suggest that we recruited HNCS who were functioning well at baseline, leaving little room for improvement in patient-reported outcomes.
Our study has important strengths and limitations. To our knowledge, the LIFTING trial is the first study to test the feasibility and safety of a HLST program in HNCS. Moreover, the HLST program was supervised and individualized. Finally, we used validated measures of health-related fitness and patient-reported outcomes.
The failure to achieve our desired sample size and track eligibility and recruitment rates are major limitations of our study that make it difficult to fully evaluate the feasibility and safety of HLST in HNCS. Furthermore, the study participants in the LIFTING trial were relatively high functioning at baseline. All participants reported a college or university education and white ethnicity and were very motived to exercise. Therefore, the findings of this study may not generalize to all HNCS. In addition, the LIFTING trial lacked a comparison group to evaluate the relative effects of HLST compared to no exercise or a LMST. Nevertheless, we felt it was crucial to conduct a phase I study to determine the feasibility and safety of HLST before initiating randomized phase II trials.
Conclusion
In conclusion, the preliminary data from the LIFTING trial suggests that a supervised HLST exercise intervention in HNCS post-surgical ND is potentially safe and feasible. Although this phase I study experienced challenges with recruitment due to the COVID-19 pandemic, it demonstrated excellent adherence, follow-up rates, minimal barriers, numerous perceived benefits, and objective strength gains in those who did participate. Phase II trials with randomized comparison groups are warranted to further examine the safety, feasibility, and efficacy of HLST in HNCS.
References
Lo Nigro C, Denaro N, Merlotti A, Merlano M (2017) Head and neck cancer: improving outcomes with a multidisciplinary approach. Cancer Manag Res 9:363–371
Al-Qurayshi Z, Walsh J, Owen S, Kandil E (2019) Surgical site infection in head and neck surgery: a national perspective. Otolaryngol Head Neck Surg 161(1):52–62
Carvalho AP, Vital FM, Soares BG (2012) Exercise interventions for shoulder dysfunction in patients treated for head and neck cancer. Cochrane Database Syst Rev 4:CD008693
Eickmeyer SM, Walczak CK, Myers KB, Lindstrom DR, Layde P, Campbell BH (2014) Quality of life, shoulder range of motion, and spinal accessory nerve status in 5-year survivors of head and neck cancer. PM R 6(12):1073–1080
Kelley MJ, Kane TE, Leggin BG (2008) Spinal accessory nerve palsy: associated signs and symptoms. J Orthop Sports Phys Ther 38(2):78–86
Popovski V, Benedetti A, Popovic-Monevska D, Grcev A, Stamatoski A, Zhivadinovik J (2017) Spinal accessory nerve preservation in modified neck dissections: surgical and functional outcomes. Acta Otorhinolaryngol Ital 37(5):368–374
Samim F, Epstein JB, Zumsteg ZS, Ho AS, Barasch A (2016) Oral and dental health in head and neck cancer survivors. Cancers Head Neck 1:14
Capozzi LC, Boldt KR, Lau H, Shirt L, Bultz B, Culos-Reed SN (2015) A clinic-supported group exercise program for head and neck cancer survivors: managing cancer and treatment side effects to improve quality of life. Support Care Cancer 23(4):1001–1007
Nayak SG, Pai MS, George LS (2016) Self-image of the patients with head and neck cancer: a mixed method research. Indian J Palliat Care 22(3):331–334
McNeely ML, Parliament MB, Seikaly H, Jha N, Magee DJ, Haykowsky MJ et al (2008) Effect of exercise on upper extremity pain and dysfunction in head and neck cancer survivors: a randomized controlled trial. Cancer 113(1):214–222
Lonbro S, Dalgas U, Primdahl H, Overgaard J, Overgaard K (2013) Feasibility and efficacy of progressive resistance training and dietary supplements in radiotherapy treated head and neck cancer patients--the DAHANCA 25A study. Acta Oncol 52(2):310–318
McGarvey AC, Osmotherly PG, Hoffman GR, Chiarelli PE (2013) Scapular muscle exercises following neck dissection surgery for head and neck cancer: a comparative electromyographic study. Phys Ther 93(6):786–797
Margaret McNeely MP, Courneya K, Haykowsky M (2004) Resistance exercise for post neck dissection shoulder pain: three case reports. Physiother Ther Pract 20:41–56
McNeely ML, Parliament M, Courneya KS, Seikaly H, Jha N, Scrimger R et al (2004) A pilot study of a randomized controlled trial to evaluate the effects of progressive resistance exercise training on shoulder dysfunction caused by spinal accessory neurapraxia/neurectomy in head and neck cancer survivors. Head Neck 26(6):518–530
McNeely ML, Parliament MB, Seikaly H, Jha N, Magee DJ, Haykowsky MJ et al (2015) Sustainability of outcomes after a randomized crossover trial of resistance exercise for shoulder dysfunction in survivors of head and neck cancer. Physiother Can 67(1):85–93
Capozzi LC, Nishimura KC, McNeely ML, Lau H, Culos-Reed SN (2015) The impact of physical activity on health-related fitness and quality of life for patients with head and neck cancer: a systematic review. Br J Sports Med 50(6):325–338
Lonkvist CK, Vinther A, Zerahn B, Rosenbom E, Deshmukh AS, Hojman P et al (2017) Progressive resistance training in head and neck cancer patients undergoing concomitant chemoradiotherapy. Laryngoscope Investig Otolaryngol 2(5):295–306
Rogers LQ, Anton PM, Fogleman A, Hopkins-Price P, Verhulst S, Rao K et al (2013) Pilot, randomized trial of resistance exercise during radiation therapy for head and neck cancer. Head Neck 35(8):1178–1188
Samuel SR, Maiya GA, Babu AS, Vidyasagar MS (2013) Effect of exercise training on functional capacity & quality of life in head & neck cancer patients receiving chemoradiotherapy. Indian J Med Res 137(3):515–520
Csapo R, Alegre LM (2016) Effects of resistance training with moderate vs heavy loads on muscle mass and strength in the elderly: a meta-analysis. Scand J Med Sci Sports 26(9):995–1006
Schoenfeld BJ, Grgic J, Ogborn D, Krieger JW (2017) Strength and hypertrophy adaptations between low- vs. high-load resistance training: a systematic review and meta-analysis. J Strength Cond Res 31(12):3508–3523
American College of Sports M (2009) American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc 41(3):687–708
Godin G, Shephard RJ (1985) A simple method to assess exercise behavior in the community. Can J Appl Sport Sci 10(3):141–146
Godin G, Kok G (1996) The theory of planned behavior: a review of its applications to health-related behaviors. Am J Health Promot 11(2):87–98
Group EQoL (2014) European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-H&N43. Phase IV Module
Tedeschi RG, Calhoun LG (1996) The Posttraumatic Growth Inventory: measuring the positive legacy of trauma. J Trauma Stress 9(3):455–471
Simard S, Savard J (2009) Fear of Cancer Recurrence Inventory: development and initial validation of a multidimensional measure of fear of cancer recurrence. Support Care Cancer 17(3):241–251
NCCN. FACT-Head & Neck Symptom Index. https://www.facit.org/facitorg/questionnaires2020 [Available from: file:///C:/Users/User/Desktop/FACITHNC.pdf.
Spielberger CD (2010) State-trait anxiety inventory. Corsini Encyclopedia of Psychology. John Wiley & Sons Inc., Hoboken
Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E (1997) Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manag 13(2):63–74
Cohen S, Kamarck T, Mermelstein R (1994) Perceived stress scale. Measuring Stress: A Guide for Health and Social Scientists 10:1–2
Taylor RJ, Chepeha JC, Teknos TN, Bradford CR, Sharma PK, Terrell JE et al (2002) Development and validation of the neck dissection impairment index: a quality of life measure. Arch Otolaryngol Head Neck Surg 128(1):44–49
AHS (2010) Guidelines for using the revised Edmonton Symptom Assessment System (ESAS-r). Seniors Health – Edmonton Zone Regional Palliative Care. Program :1–7
Jordan CH (2020) Rosenberg self-esteem scale. In: Zeigler-Hill V, Shackelford TK (eds) Encyclopedia of Personality and Individual Differences. Springer, Cham. https://doi.org/10.1007/978-3-319-24612-3_1155
Morin CM, Belleville G, Belanger L, Ivers H (2011) The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 34(5):601–608
Cešeiko R, Thomsen SN, Tomsone S, Eglītis J, Vētra A, Srebnijs A, Timofejevs M, Purmalis E, Wang E (2020) Heavy resistance training in breast cancer patients undergoing adjuvant therapy. Medicine and Science in Sports and Exercise 52(6):1239–1247. https://doi.org/10.1249/MSS.0000000000002260
Funding
KSC is supported by the Canada Research Chairs Program and a Foundation Grant (#159927) from the Canadian Institutes of Health Research
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by SMN, MLM, and KSC. The first draft of the manuscript was written by SMN, and all authors reviewed previous versions of the manuscript. The authors read and approved of the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was performed in line with the Principles of the Declaration of Helsinki. Approval was granted by the Health Research Ethics Board of Alberta-Cancer Committee (HREBA-CC).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
All authors in the study have agreed on the publication of this manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1:
Figure S1. Typical Intensity Progressions in the LIFTING Trial (JPG 65 kb).
ESM 2:
Maximal strength testing protocol. Table S1. Resistance Training Parameters. Table S2. 12 Week HLST Exercise Program Intensities for Main Movements. Table S3. 12 Week Exercise Program Intensities for Accessory Movements. Table S4. HLST in Cancer Populations. Table S5. Exercise Interventions in HNC Populations. Table S6. Feasibility of Baseline Maximal Strength Testing in the LIFTING Trial. Table S7. Feasibility of Heavy LIFTING Strength Training in the LIFTING Trial. Table S8. Perceived Benefits of Heavy Lifting Strength Training in the LIFTING Trial. Table S9. Changes in Motivational and Behavioural Outcomes from Baseline to Postintervention in the LIFTING Trial. Table S10. Changes in EORTC QLQ-C30 from Baseline to Postintervention in the LIFTING Trial. Table S11. Changes in Patient-Reported Symptom and Psychosocial Outcomes from Baseline to Postintervention in the LIFTING Trial (DOCX 58 kb).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ntoukas, S.M., McNeely, M.L., Seikaly, H. et al. Feasibility and safety of Heavy Lifting Strength Training in Head and Neck Cancer survivors post-surgical neck dissection (the LIFTING trial). Support Care Cancer 31, 348 (2023). https://doi.org/10.1007/s00520-023-07815-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-07815-2