Abstract
The recycling of lithium-ion batteries (LIBs) is currently an important topic in the fields of research and industry. New developments for the recovery of valuable metals from spent LIBs show a clear trend towards hydrometallurgical concepts. In this context, pre-treatment in particular plays an essential role. If organic compounds remain in the material or if the cathode and anode materials are insufficiently separated from the conductor foils, this can lead to massive process complications in the subsequent hydrometallurgical processes. The chair of Non-ferrous Metallurgy is develo** and testing the adaptation and improvement of the recycling possibilities of used lithium-ion batteries for subsequent hydrometallurgical processing. Particular attention is paid to the use of synergy effects and the interaction of treatment and metallurgical processes as this combination is the only way to find an efficient and economical way to recover valuable metals. For this purpose, the comparison of different aggregates for the optimal preparation of the active material from spent lithium-ion batteries for subsequent further processing is carried out. By improving the overall processes, the recovery rates for the valuable metals contained, such as cobalt, nickel, and lithium, can be significantly increased, the metallurgical processes be optimised and the raw material cycle be closed. This is a significant contribution to environmental and climate protection, especially in view of the criticality of the elements mentioned. Due to the demand for a holistic solution for the long-term supply of the European Union with critical raw materials through recycling, a significant optimisation in the field of recovering valuable metals from used lithium-ion batteries can be realised.
Zusammenfassung
Das Recycling von Lithium-Ionen-Batterien (LIBs) stellt aktuell eine wichtige Thematik im Bereich der Forschung und Industrie dar. Neue Entwicklungen zur Rückgewinnung von wertvollen Metallen aus verbrauchten LIBs zeigen einen klaren Trend zu hydrometallurgischen Konzepten. In diesem Zusammenhang spielt vor allem die Vorbehandlung eine wesentliche Rolle. Verbleiben organische Verbindungen im Material oder werden die Kathoden- und Anodenmaterialien nur unzureichend von den Leiterfolien getrennt, kann dies zu massiven Prozesskomplikationen in den nachfolgenden hydrometallurgischen Verfahren führen. Der Lehrstuhl für Nichteisenmetallurgie entwickelt und erprobt daher die Anpassung und Verbesserung der Recyclingmöglichkeiten von gebrauchten Lithium-Ionen-Batterien für eine anschließende hydrometallurgische Weiterverarbeitung. Besonderes Augenmerk wird dabei auf die Nutzung von Synergieeffekten und das Zusammenwirken von Aufbereitungs- und metallurgischen Verfahren gelegt, da nur durch diese Kombination ein effizienter und wirtschaftlicher Weg zur Rückgewinnung von Wertmetallen beschritten werden kann. Zu diesem Zweck erfolgt der Vergleich verschiedener Aggregate zur optimalen Aufbereitung des Aktivmaterials aus verbrauchten Lithium-Ionen-Batterien für die anschließende Weiterverarbeitung. Durch die Verbesserung der Gesamtverfahren können die Rückgewinnungsgrade für die enthaltenen Wertmetalle wie Kobalt, Nickel und Lithium deutlich erhöht, die metallurgischen Prozesse optimiert sowie damit der Rohstoffkreislauf geschlossen werden. Dies ist ein wesentlicher Beitrag zum Umwelt- und Klimaschutz, insbesondere angesichts der Kritikalität der angesprochenen Elemente. Durch den Anspruch einer ganzheitlichen Lösung für die langfristige Versorgung der Europäischen Union mit kritischen Rohstoffen durch Recycling kann somit eine wesentliche Optimierung im Bereich der Rückgewinnung von wertvollen Metallen aus verbrauchten Lithium-Ionen-Batterien realisiert werden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
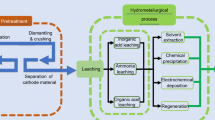
1 Pre-processing of Spent Lithium-ion Batteries
The move towards electromobility and the use of lithium-ion batteries in various electronic devices has recently led to an immense increase in LIBs becoming waste [1]. This trend will continue in the coming years as a demand for 25.8–46.8 million electric vehicles is expected by 2030 [2]. This consideration does not take into account smaller cells such as those used in smartphones or laptops. Based on this situation, the need for efficient recycling processes for the resulting lithium-ion batteries at the end of their life span can be quickly identified. Different approaches can already be found on the market, whereby a trend can already be identified here as well. It is becoming apparent that the hydrometallurgical recycling route in particular will be highly relevant in the future as this enables selective multi-metal recycling. In contrast, valuable metals such as lithium or manganese are slagged in pyrometallurgical methods, which removes them from the circular economy. When using hydrometallurgical processes, however, optimal preparation of the battery materials in advance is essential. Otherwise, there will be poor yields in terms of recycling rates and only low-quality secondary products. The combination of pre-treatment processes and hydrometallurgy, in particular, is currently hardly discussed in detail in the literature as the two disciplines are often considered separately. This is an important point, especially due to the complexity of the dependency of the mentioned specialties. An unsuitable combination of preparation steps and subsequent hydrometallurgy can lead to a complete failure of the process, whereby a large number of parameters can be decisive [3,4,5].
1.1 Sorting and Disassembly
Cell shapes and cell chemistries do not have a standardised design in either the electric vehicle or in the consumer sector. This makes the automation and description of recycling processes for spent lithium-ion batteries extremely difficult as different recycling routes are used for various cell chemistries. Mixing different battery types has a negative impact on the subsequent product quality of the secondary materials. The EU Battery Directive 2006/66/EC aims to give detailed information on battery components and cell chemistries [5]. The implementation of this directive should lead to a so-called “battery passport” and enable safer handling and high-quality recycling. Current separation processes consist of different sections, such as visual sorting, magnetic separation, X‑ray, electromagnetic, and UV-sorting. However, the automation of the processes is very difficult due to the different input parameters, such as size, weight, and design, and therefore leads to high handling costs. Appropriate labelling of the batteries is therefore absolutely necessary and indispensable for the automation of pre-sorting and material separation. After this pre-sorting, appropriate disassembling can take place. Reusable materials, such as steel, copper, aluminium, selected plastics, and, in some cases, precious metals, can be recovered from the housing, cable harness, cooling system, or other electronic parts. This also leads to a reduction in the volume flow and the variety of materials. However, this process step is also very closely linked to intensive time and personnel expenditure as currently every EV battery has to be disassembled manually and the material used requires special training and tools due to the high voltage range and high energy density. The choice of disassembly depth influences the further downstream recycling route as either cells or modules are used as input for further processes. The definition of guidelines for eco-friendly battery design would therefore both simplify the automation of the dismantling process and reduce costs accordingly [5, 6].
1.2 Discharging and Deactivation
Dismantling should allow easy discharging and diagnosis of the current state-of-health of the modules as this information is essential for possible further use of the battery. Currently, there are two ways to discharge a cell on the market: On the one hand, there is the possibility of discharging with an ohmic resistor and, on the other hand, through the application of an electrically conductive liquid, whereby the latter variant is only used for smaller cells. Very often, deactivation by means of pyrolysis takes place instead of or in combination with discharging. Lithium-ion batteries are normally applied in a pyrolysis process before mechanical pre-treatment takes place. This leads to a deactivation of the cells mainly for safety reasons. The energy content is significantly reduced during the process in a controlled manner and the organic components are removed. In addition, halogenated substances are discharged via the exhaust gas. The removal of the organic binder results in the detachment of the active material from the copper and aluminium foils, whereby a first stage in the separation of impurities for further hydrometallurgical processing can be realised. Furthermore, the separation of organic components is the first step towards a trouble-free hydrometallurgical processing of the material as organic components in aqueous solutions lead to a deterioration in leaching efficiency and wettability of the active material. After the pyrolysis process, the batteries can be stored temporarily and be mechanically processed at low risks of fire or thermal runaways. This process is carried out at 500–600 °C with a nitrogen purge to prevent the input material from burning off. The temperature can have a significant impact on process efficiency as temperatures above 600 °C lead to aggregation and larger amounts of pyrolysis residues. In this case in particular, the exhaust gas side must be taken into account as fluorine-containing beneze and esters from the electrolyte are found in the waste gas during the decomposition of the plastics and organic solvents [7,8,9].
1.3 Mechanical Pre-treatment
Before using the active material in metallurgical processes, a mechanical pre-treatment of the batteries is indispensable in order to be able to present an efficient process. The main task is to separate the valuable output fractions (Fe, Cu, Al) from the fine material (active material). The latter is to be further processed in downstream metallurgical processes in order to recover Li, Co, Ni, Mn, and graphite. There are different approaches to mechanical comminution. Both the atmospheres (inert via N2 purging or ambient atmosphere) and the number of process steps differ. When using two comminution steps, a slow-running (e.g. low-speed rotary mill) and subsequently a fast-running unit such as a high-speed impact mill can be used. In addition to the already mentioned aggregates, hammer mills are also frequently used in various modifications. Particularly excellent results in terms of the percentage of recovered electrode material could be achieved when using hammer crushing with a two-blade rotor crusher. When comparing dry and wet crushing methods, dry crushing, which was conducted in a two-stage process, proved to be particularly advantageous as the electrode materials could be separated from the Al and Cu foils without overcrushing other components from the spent battery. By using a shear crusher, the metal shell could be comminuted and the electrode material recovered, while the subsequent impact crushing after an intermediate dry sieving process achieved an appropriate size reduction for further downstream metallurgical processes [10,11,12].
The comminution steps are followed by the separation of metal and foil components from the electrode material. This can also be realised by different processes. Aggregates such as magnetic separators and air classification are used for this purpose. For the subsequent screening, the mesh sizes are classically in the range of 250 µm to 2 mm as this guarantees an almost complete separation of the foil components and the finer fractions. In the literature, some attempts can be found to selectively separate the electrode material from other components (Fe, Al, Cu) as this appears to be particularly advantageous for further hydrometallurgical processing. Viececli et al. have shown that, after shredding the cells, a sieve cut at approx. 6 mm proves to be useful as this allows the majority of the iron fraction to be separated. This is due to the fact that iron is more resistant against shredding. The second sieve cut was set at 2 mm in order to separate foil components made of copper and aluminium from the electrode material. The remaining fraction with a grain size below 2 mm contains the cathode and anode materials and can be optimally processed in hydrometallurgy [13].
This stage is followed by appropriate separation processes for the different fractions. Magnetic, eddy current, electrostatic, gravity separation, and froth flotation can be used to classify the comminution products, which were previously only classified on the basis of their grain size. Further processing is not discussed in this contribution as the focus is primarily on the hydrometallurgical processing of the obtained fine fraction [5, 14].
2 Experimental
Within the framework of the conducted tests, the discharge of spent lithium-ion batteries by means of ohmic resistance, the opening as well as the removal of the steel casing by means of sheat shears, and the extraction of the battery pack were carried out. This was deactivated in a pyrolysis process at 500 °C for 5 h. The entire battery pack was fed to the LITech HM 200 hammer mill (see Fig. 1a) in its entirety. After a process time of only a few seconds at a speed of 3000 rpm, an appropriate comminution of the material was achieved. The same procedure was carried out for the LITech RM 250 rotor mill (see Fig. 1b). At a speed of 3000 rpm, the time required for the corresponding comminution was significantly increased and coarser particles remained in the rotor mill discharge.
The material obtained from the two comminution units was fed to a sieve with sieve sections at 4 mm, 1 mm, and 500 µm. The corresponding documentation of the fractions obtained can be seen in Fig. 2 exemplary for the tests with the hammer mill. The last fraction (< 500 µm) was then applied for further hydrometallurgical processing in an acid leaching and the relevant parameters were qualitatively checked. In addition, a particle size distribution of the mechanically obtained fine fraction was also determined and the separation of the electrode materials was verified using digital microscopy (Keyence VHX-7000) and scanning electron microscopy (JEOL JSM-IT300).
2.1 Hydrometallurgical Processing
For subsequent hydrometallurgical processes in general and for leaching in particular, several influencing factors can be defined. These include the wettability of the active material used, the potential for foam formation and the height of the foam layer formed in the reactor, the start-up and long-term behaviour of the process, the flowability of the input material, and also the filterability after leaching. The parameters mentioned are not a complete enumeration of the influencing variables for the process, but include the most important points for an initial consideration, especially with regard to the preceding treatment. Based on the parameters of this list and the quality of the processed electrode material, the suitability of different aggregates for crushing of the battery pack without housing is investigated in this publication. As described, a large number of possibilities and implementations can be found in the literature for the first comminution step, which mainly uses slow-running aggregates to comminute the enclosure of the cell. For the second comminution step, high-speed aggregates are frequently applied, although no optimum has yet been found here, especially in consideration of the subsequent specifications for the hydrometallurgical process. Within the scope of the research work carried out, two essential aggregates for secondary comminution could be tested. These include a LITech hammer mill (HM 200) with serrated hammers and a LITech rotor mill (RM 250). The considerations include, on the one hand, the effort for the actual comminution and the possibilities for the subsequent preparation and hydrometallurgy, on the other hand.
3 Results and Discussion
In the following subsections, a qualitative analysis of the tested processing steps will be conducted. Since it was already evident at this early stage of the research that the rotor mill leads to significantly longer process times and a qualitatively and already visually recognisable poorer comminution, the further evaluations will focus on those fractions from the pre-treatment with the hammer mill. In addition, when using this aggregate, large amounts of active material remain on the arrester foils. This behaviour would lead to enormous losses in the area of recycling rates.
3.1 Microscopic Analysis of the Obtained Fractions
In order to be able to estimate the efficiency of the pre-treatment as well as possible material losses due to adhesions of the active material to cathode and anode foils, the microscopic recording is carried out in the digital as well as scanning electron microscope (see Fig. 3). It can be proved that individual remnants of active material can be seen on both the cathode and the anode foil of the fraction above 4 mm, but the majority can be separated by means of comminution in the hammer mill and subsequent sieving.
Hardly any anode material can be detected on the foil components of the fraction 1–4 mm, while individual Al foil components certainly show residues of cathode material. In this case the problem of incomplete separation of the organic binder can be estimated. Optical sorting of anode and cathode arrester foils is no longer possible from a grain size of less than 1 mm. The SEM/EDS images show mixed foil sections and residues of adhering active material (see Fig. 3). In the last fraction (< 0.5 mm), the majority of the active material can be detected. Furthermore, hardly any film components are detected in this fraction, making it ideally suitable for further hydrometallurgical processing. The element distribution also clearly shows the presence of a mixed oxide.
From this evaluation it can be concluded that a qualitatively sufficient separation of the cathode and anode material from the arrester foils is possible by processing in the hammer mill with serrated hammer due to the combined impact and shearing effect of the exposure in this unit in combination with subsequent sieving. A further consideration of the fraction from 0.5 to 1 mm is still pending as in this case a further pre-processing step may be necessary to minimise the risk of material loss.
3.2 Sieve Analysis of the Obtained Active Material
The following section shows the evaluation of the sieve analysis carried out for the fraction of the active material obtained starting with the sieve size of 1 mm. For this analysis, an amplitude of 0.7 mm and a sieving time of 3 min were applied. The evaluation resulted in characteristic values for the Sauter diameter of d32 = 41.99 µm and d50 = 53 µm. Figure 4 shows the corresponding frequency distribution and the relative frequency.
3.3 Qualitative Assessment of the Leaching Capacity of the Obtained Active Material
When considering the relevant criteria for leaching behaviour qualitatively, the following results can be identified for the obtained active material. The wettability can be classified as good as only a small percentage of the particles float on the surface of the leaching solution. The corresponding measurement of foam formation showed a height of approx. 15–20 mm, whereby this is mainly caused by the production of gaseous CO2 and only a few particles float up. These particles are platelet-shaped and therefore most likely represent portions of the anode material respectively graphite. This fact leads to the conclusion that the relevant parts of the active material (nickel-manganese-cobalt oxide) are sufficiently wetted and thus available for leaching. The start-up behaviour of the process shows itself to be quite reactive but manageable through targeted temperature control. This enables the addition of the active material without having to accept a possible runaway of the reaction. This statement applies not only to leaching with low concentrated sulphuric acid (max. 2 molar), but also to a process with the slow addition of up to 5 vol.% H2O2, which is often used as an oxidising agent in classical hydrometallurgical processes. When adding hydrogen peroxide, particular attention must be paid to the formation of foam due to the release of oxygen as this foam building behaviour can be intensified. The stirability of the solution can be confirmed, whereby in this context solid-liquid ratios between 50 and 120 g of solid material per litre of leaching solution were checked. This parameter has so far only been verified on a small scale using a magnetic stirring plate and associated magnetic rod. If higher solid-liquid ratios are required for further processing, an external stirring could be alternatively realised. Since the active material is dry and the foil components were sufficiently separated as described, the parameter of flowability also proved to be unproblematic. In the area of subsequent filtration after leaching, the test was carried out by means of vacuum filtration, which was feasible for a defined sample quantity in a short time (< 60 s). This indicates that no over-comminution of the particles took place, which would lead to extremely small grains that would clog the filter materials and thus corresponding process times can be observed.
4 Conclusion and Summary
In summary, the comminution of spent lithium-ion batteries is a challenging discipline, especially depending on the subsequent downstream metallurgical processes. In the comminution tests for the battery pack with a previous removal of the case as well as a pyrolysis for safety reasons with rotor and hammer mills, it was shown that a combined impact and shear stress as applied by a hammer mill with serrated hammer (LITech HM 200) offers great advantages. In addition to the shorter process times for comminution, it also proved better separation of the active materials from the foil components and thus a higher yield. The qualitative analysis of selected hydrometallurgical relevant parameters for the electrode material stream of the hammer mill showed that no over-comminution took place and that the reaction behaviour can also be judged quite positively. For further tests, quantitative leaching experiments will have to be carried out in order to show possible influences of the comminution on the specific leaching efficiency of individual elements. In addition, the separation of electrode material for the grain size class 0.5–1 mm needs to be optimized with a view to avoid material losses and to recover a pure and recyclable foil fraction.
References
European Commission: Regulation of the European Parliament and the Council concerning batteries and waste batteries, repealing Directive 2006/66/EC and amending regulation (EU) No2019/1020 (2020). https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52020PC0798, Accessed 15 May 2023
IEA: Global EV Sales by Scenario 2020–2030 (2022). https://www.iea.org/data-and-statistics/charts/global-ev-salesby-scenario-2020-2030, Accessed 15 May 2023
Georgi-Maschler, T., Friedrich, B., Weyhe, R., Heegn, H., Rutz, M.: Development of a recycling process for Li-ion batteries. J Power Sources 207, 173–182 (2012)
Hanisch, C., Loellhoeffel, T., Diekmann, J., Markley, K.J., Haselrieder, W., Kwade, A.: Recycling of lithium-ion batteries: a novel method to separate coating and foil of electrodes. J Clean Prod 108, 301–311 (2015)
Martens, H., Goldmann, D.: Recyclingtechnik. Springer, Wiesbaden (2016)
Sziegoleit, H.: Sortierung von Gerätebatterien. Recycling und Rohstoffe. TK-Verlag, Neuruppin (2013)
Rallo, H., Benveniste, G., Gestoso, I., Amante, B.: Economic analysis of the disassembling activities to the reuse of electric vehicles Li-ion batteries. Resour. Conserv. Recycl. 159, 104785 (2020)
Gentilini, L., Mossali, E., Angius, A., Colledani, M.: A safety oriented decision support tool for the remanufacturing and recycling of post-use H&EVs Lithium-Ion batteries. Proc CIRP , 73–78 (2020). https://doi.org/10.1016/j.procir.2020.01.090
Brückner, L., Frank, J., Elwert, T.: Industrial recycling of lithium-Ion batteries—A critical review of metallurgical process routes. Metals 10, 1107 (2020)
Velázquez-Martínez, Valio, Santasalo-Aarnio, Reuter, Serna-Guerrero: A critical review of lithium-Ion battery recycling processes from a circular economy perspective. Batteries 5, 68 (2019)
Diekmann, J., Hanisch, C., Froböse, L., Schälicke, G., Loellhoeffel, T., Fölster, A.-S., Kwade, A.: Ecological recycling of lithium-Ion batteries from electric vehicles with focus on mechanical processes. J. Electrochem. Soc. 164, A6184 (2017)
Windisch-Kern, S., Gerold, E., Nigl, T., Jandric, A., Altendorfer, M., Rutrecht, B., Scherhaufer, S., Raupenstrauch, H., Pomberger, R., Antrekowitsch, H., Part, F.: Recycling chains for lithium-ion batteries: A critical examination of current challenges, opportunities and process dependencies. Waste Manag. 138, 125–139 (2022)
Vieceli, N., Nogueira, C.A., Guimaraes, C., Pereira, M.F.C., Durao, F.O., Margarido, F.: Hydrometallurgical recycling of lithium-ion batteries by reductive leacing with sodium metabisulphite. Waste Manag. 71, 350–361 (2018)
Gratz, E., Sa, Q., Apelian, D., Wang, Y.: A closed loop process for recycling spent lithium ion batteries. J Power Sources 262, 255–262 (2014)
Funding
Open access funding provided by Montanuniversität Leoben.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gerold, E., Anbauer, A., Kügele, A. et al. Interrelationships between Pre-processing and Subsequent Procedures in the Recycling of Spent Lithium-ion Batteries. Berg Huettenmaenn Monatsh 168, 346–352 (2023). https://doi.org/10.1007/s00501-023-01361-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00501-023-01361-4
Keywords
- Recycling
- Spent lithium-ion batteries
- Pre-processing
- Mechanical processing
- Hydrometallurgy
- Process development