Abstract
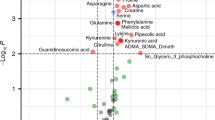
An early prediction of outcomes of neonatal hypoxic-ischemic encephalopathy (NE) is of key importance in reducing neonatal mortality and morbidity. The objectives were (i) to analyze the characteristics of miRNA expression and metabolic patterns of neonates with NE and (ii) to assess their predictive performance for neurodevelopmental outcomes. Plasma samples from moderate/severe NE patients (N = 92) of the HYPOTOP study were collected before, during, and after therapeutic hypothermia (TH) and compared to a control group (healthy term infants). The expression of miRNAs and concentrations of metabolites (hypoxia-related and energy, steroid, and tryptophan metabolisms) were analyzed. Neurodevelopmental outcomes were evaluated at 24 months postnatal age using Bayley Scales of Infant Development, ed. III, BSID-III. Differences in miRNA and metabolic profiles were found between NE vs. control infants, abnormal (i.e., mildly and moderately abnormal and severe) vs. normal, and severe vs. non-severe (i.e., normal and mildly and moderately abnormal) BSID-III. 4-Androstene-3,17-dione, testosterone, betaine, xanthine, and lactate were suitable for BSID-III outcome prediction (receiver operating characteristic areas under the curve (AUCs) ≥ 0.6), as well as 68 miRNAs (AUCs of 0.5–0.9). Significant partial correlations of xanthine and betaine levels and the expression of several miRNAs with BSID-III sub-scales were found.
Conclusion: We have identified metabolites/miRNAs that might be useful to support the prediction of middle-term neurodevelopmental outcomes of NE.
What is known and what is new: |
|---|
• The early prediction of outcomes of neonatal hypoxic-ischemic encephalopathy (NE) is of key importance in reducing neonatal mortality and morbidity. |
• Alterations of the metabolome and miRNAs had been observed in NE. |
• We performed miRNA sequencing and quantified selected metabolites (i.e., lactate, pyruvate, ketone bodies, Krebs cycle intermediates, tryptophan pathway, hypoxia-related metabolites, and steroids) by GC- and LC–MS. |
• Specific miRNAs and metabolites that allow prediction of middle-term neurodevelopmental outcomes of newborns with NE undergoing hypothermia treatment were identified. |



Similar content being viewed by others
Data availability
All data generated during this study are included in supplementary information files of this published article.
References
Wisnowski JL, Wintermark P, Bonifacio SL et al (2021) Neuroimaging in the term newborn with neonatal encephalopathy. Semin Fetal Neonatal Med 26:101304. https://doi.org/10.1016/j.siny.2021.101304
Wassink G, Davidson JO, Dhillon SK et al (2019) Therapeutic hypothermia in neonatal hypoxic-ischemic encephalopathy. Curr Neurol Neurosci Rep 19:2. https://doi.org/10.1007/s11910-019-0916-0
Midan DAR, Bahbah WA, Fayed DA et al (2021) Cord blood microRNA-376c and microRNA-1268a as biomarkers for neonatal hypoxic-ischaemic encephalopathy: a diagnostic accuracy study. BMJ Paediatrics Open 5:e001258. https://doi.org/10.1136/bmjpo-2021-001258
Ma Q, Zhang L (2015) Epigenetic programming of hypoxic-ischemic encephalopathy in response to fetal hypoxia. ProgNeurobiol 0:28–48. https://doi.org/10.1016/j.pneurobio.2014.11.001
Wassink G, Harrison S, Dhillon S et al (2022) Prognostic neurobiomarkers in neonatal encephalopathy. Dev Neurosci 44:331–343. https://doi.org/10.1159/000522617
Dakroub F, Kobeissy F, Mondello S et al (2024) MicroRNAs as biomarkers of brain injury in neonatal encephalopathy: an observational cohort study. Sci Rep 14:6645. https://doi.org/10.1038/s41598-024-57166-z
Piñeiro-Ramos JD, Núñez-Ramiro A, Llorens-Salvador R et al (2020) Metabolic phenotypes of hypoxic-ischemic encephalopathy with normal vs. pathologic magnetic resonance imaging outcomes. Metabolites 10:109. https://doi.org/10.3390/metabo10030109
Piñeiro-Ramos JD, Cascant MM, Núñez-Ramiro A et al (2022) Noninvasive monitoring of evolving urinary metabolic patterns in neonatal encephalopathy. Pediatr Res 91:598–605. https://doi.org/10.1038/s41390-021-01553-z
Locci E, Noto A, Puddu M et al (2018) A longitudinal 1H-NMR metabolomics analysis of urine from newborns with hypoxic-ischemic encephalopathy undergoing hypothermia therapy. Clinical and medical legal insights. PLOS ONE 13:e0194267. https://doi.org/10.1371/journal.pone.0194267
Ahearne C, Denihan N, Walsh B et al (2016) Early cord metabolite index and outcome in perinatal asphyxia and hypoxic-ischaemic encephalopathy. Neonatology 110:296–302. https://doi.org/10.1159/000446556
Sánchez-Illana Á, Solberg R, Lliso I et al (2017) Assessment of phospholipid synthesis related biomarkers for perinatal asphyxia: a piglet study. Sci Rep 7:40315. https://doi.org/10.1038/srep40315
Piñeiro-Ramos JD, Núñez-Ramiro A, Llorens-Salvador R, et al (2020) Metabolic phenotypes of hypoxic-ischemic encephalopathy with normal vs. Pathologic Magnetic Resonance Imaging Outcomes. Metabolites 10:. https://doi.org/10.3390/metabo10030109
Nuñez-Ramiro A, Benavente-Fernández I, Valverde E et al (2019) Topiramate plus cooling for hypoxic-ischemic encephalopathy: a randomized, controlled, multicenter, double-blinded trial. Neonatology 116:76–84. https://doi.org/10.1159/000499084
Sarnat HB (1976) Neonatal encephalopathy following fetal distress: a clinical and electroencephalographic study. Arch Neurol 33:696. https://doi.org/10.1001/archneur.1976.00500100030012
Shankaran S, McDonald SA, Laptook AR et al (2015) Neonatal magnetic resonance imaging pattern of brain injury as a biomarker of childhood outcomes following a trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J Pediatr 167:987-993.e3. https://doi.org/10.1016/j.jpeds.2015.08.013
Bayley Scales of Infant and Toddler Development | Third Edition. https://www.pearsonassessments.com/store/usassessments/en/Store/Professional-Assessments/Behavior/Adaptive/Bayley-Scales-of-Infant-and-Toddler-Development-%7C-Third-Edition/p/100000123.html. Accessed 7 Apr 2022
Sánchez-Illana Á, Núñez-Ramiro A, Cernada M et al (2017) Evolution of energy related metabolites in plasma from newborns with hypoxic-ischemic encephalopathy during hypothermia treatment. Sci Rep 7:1–12. https://doi.org/10.1038/s41598-017-17202-7
Lario S, Ramírez-Lázaro MJ, Sanjuan-Herráez D et al (2017) Plasma sample based analysis of gastric cancer progression using targeted metabolomics. Sci Rep 7:1–10. https://doi.org/10.1038/s41598-017-17921-x
O’Sullivan MP, Looney AM, Moloney GM et al (2019) Validation of altered umbilical cord blood microRNA expression in neonatal hypoxic-ischemic encephalopathy. JAMA Neurol 76:333–341. https://doi.org/10.1001/jamaneurol.2018.4182
Wang Z, Liu Y, Shao M et al (2018) Combined prediction of miR-210 and miR-374a for severity and prognosis of hypoxic–ischemic encephalopathy. Brain Behav 8:e00835. https://doi.org/10.1002/brb3.835
Li M, Ye M, Zhang G (2021) Aberrant expression of miR-199a in newborns with hypoxic-ischemic encephalopathy and its diagnostic and prognostic significance when combined with S100B and NSE. Acta Neurol Belg 121:707–714. https://doi.org/10.1007/s13760-020-01408-0
Friedes BD, Molloy E, Strickland T et al (2021) Neonatal encephalopathy plasma metabolites are associated with neurodevelopmental outcomes. Pediatr Res 1–8. https://doi.org/10.1038/s41390-021-01741-x
Kleinman HK, Philp D, Hoffman MP (2003) Role of the extracellular matrix in morphogenesis. Curr Opin Biotechnol 14:526–532. https://doi.org/10.1016/j.copbio.2003.08.002
Zalewska T, Makarewicz D, Janik B, Ziemka-Nałęcz M (2005) Neonatal cerebral hypoxia-ischemia: involvement of FAK-dependent pathway. Int J Dev neurosci 23:657–662. https://doi.org/10.1016/j.ijdevneu.2005.05.010
Dai J, Wang J, Yang L et al (2015) miR-125a regulates angiogenesis of gastric cancer by targeting vascular endothelial growth factor A. Int J Oncol 47:1801–1810. https://doi.org/10.3892/ijo.2015.3171
Aly H, Hassanein S, Nada A et al (2009) Vascular endothelial growth factor in neonates with perinatal asphyxia. Brain Dev 31:600–604. https://doi.org/10.1016/j.braindev.2008.09.004
Torun D, Arslan M, Yüksel Z (2021) Coexistence of severe developmental delay, epilepsy, and hemangioma in Snijders Blok-Fisher syndrome suggests the presence of a POU3F3-related SNIBFIS endophenotype: a case report. Am J Med Genet A 185:1554–1560. https://doi.org/10.1002/ajmg.a.62135
Watts D, Stein J, Meneses A et al (2021) HIF1α is a direct regulator of steroidogenesis in the adrenal gland. Cell Mol Life Sci 78:3577–3590. https://doi.org/10.1007/s00018-020-03750-1
Solberg R, Escobar J, Arduini A et al (2013) Metabolomic analysis of the effect of postnatal hypoxia on the retina in a newly born piglet model. PLoS ONE 8:e66540. https://doi.org/10.1371/journal.pone.0066540
Solberg R, Kuligowski J, Pankratov L et al (2016) Changes of the plasma metabolome of newly born piglets subjected to postnatal hypoxia and resuscitation with air. Pediatr Res 80:284–292. https://doi.org/10.1038/pr.2016.66
Skappak C, Regush S, Cheung P-Y, Adamko DJ (2013) Identifying hypoxia in a newborn piglet model using urinary NMR metabolomic profiling. PLoS ONE 8:e65035. https://doi.org/10.1371/journal.pone.0065035
Kuligowski J, Solberg R, Sánchez-Illana Á et al (2017) Plasma metabolite score correlates with hypoxia time in a newly born piglet model for asphyxia. Redox Biol 12:1–7. https://doi.org/10.1016/j.redox.2017.02.002
O’Boyle DS, Dunn WB, O’Neill D et al (2021) Improvement in the prediction of neonatal hypoxic-ischemic encephalopathy with the integration of umbilical cord metabolites and current clinical makers. J Pediatr 229:175-181.e1. https://doi.org/10.1016/j.jpeds.2020.09.065
Cascant-Vilaplana MM, Lara-Cantón I, Núñez-Ramiro A, et al (2023) Longitudinal perturbations of plasma nuclear magnetic resonance profiles in neonatal encephalopathy. Pediatr Res 1–10. https://doi.org/10.1038/s41390-023-02464-x
Reinke SN, Walsh BH, Boylan GB et al (2013) 1H NMR derived metabolomic profile of neonatal asphyxia in umbilical cord serum: implications for hypoxic ischemic encephalopathy. J Proteome Res 12:4230–4239. https://doi.org/10.1021/pr400617m
Saugstad OD (2010) Resuscitation of newborn infants: from oxygen to room air. Lancet 376:1970–1971. https://doi.org/10.1016/S0140-6736(10)60543-0
Acknowledgements
The authors would like to express their gratitude to the parents and their newborns who participated in the study.
HYPOTOP study group
Ana Gimeno2, María Gormaz2, Raquel Escrig2, María Cernada2, Marta Aguar2, Antonio Núñez-Ramiro2, Isabel Benavente-Fernández7, Eva Valverde8, Malaika Cordeiro8, Dorotea Blanco9, Hector Boix10, Fernando Cabañas11, Mercedes Chaffanel12, Belén Fernández-Colomer13, Jose Ramón Fernández-Lorenzo14, Begoña Loureiro15, Maria Teresa Moral-Pumarega16, Antonio Pavón17, and Inés Tofé18
2Division of Neonatology, University & Polytechnic Hospital La Fe, Valencia, Spain
7Division of Neonatology, University Hospital Puerta del Mar, Cádiz, Spain
8Division of Neonatology, University Hospital La Paz, Madrid, Spain
9Division of Neonatology, University Hospital Gregorio Marañón, Madrid, Spain
10Department of Neonatology, University Hospital Vall d’Hebrón, Barcelona, Spain
11Division of Neonatology, University Hospital Quirónsalud Madrid, Madrid, Spain
12Division of Neonatology, Regional University Hospital Málaga, Málaga, Spain
13Division of Neonatology, Central University Hospital of Asturias, Oviedo, Spain
14Division of Neonatology, University Hospital Complex of Vigo, Vigo, Spain
15Division of Neonatology, University Hospital Cruces, Bilbao, Spain
16Division of Neonatology, University Hospital 12 de Octubre, Madrid, Spain
17Division of Neonatology, University Hospital Virgen del Rocío, Sevilla, Spain
18Division of Neonatology, University Hospital Reina Sofía, Córdoba, Spain
Funding
This work was supported by the Instituto de Salud Carlos III (ISCIII), Spain (grant numbers CM20/00187, CPII21/00003, EC11-046, and PI20/00964), and co-funded by the European Union. This study has been funded by Instituto de Salud Carlos III (ISCIII) through the project “RD21/0012/0015” and co-funded by the European Union—NextGenerationEU.
Author information
Authors and Affiliations
Consortia
Contributions
M.M.C.-V.: Methodology, Software, Validation, Formal analysis, Investigation, Data Curation, Writing—Original Draft, Writing—Review & Editing, Visualization. J.D.P.-R.: Methodology, Investigation, Data Curation, Writing—Review & Editing. ASG: Methodology, Investigation, Data Curation, Writing—Review & Editing. I.L.-C.: Methodology, Investigation, Data Curation, Writing—Review & Editing. Isabel Izquierdo: Methodology, Investigation, Resources, Writing—Review & Editing, Supervision. R.L.: Methodology, Formal analysis, Investigation, Data Curation, Writing—Review & Editing. P.M.: Methodology, Investigation, Writing—Review & Editing. E.T.: Methodology, Investigation, Writing—Review & Editing. C.M.: Methodology, Software, Formal analysis, Data Curation, Writing—Review & Editing. F.M.: Conceptualization, Software, Resources, Writing—Review & Editing, Supervision. N.B.: Methodology, Investigation, Data Curation, Writing—Review & Editing. G.Q.: Conceptualization, Software, Investigation, Data Curation, Writing—Review & Editing. J.K.: Conceptualization, Software, Formal analysis, Investigation, Resources, Data Curation, Writing—Original Draft, Writing—Review & Editing, Visualization, Supervision, Project administration. M.V.: Conceptualization, Resources, Writing—Review & Editing, Supervision, Project administration.
Corresponding author
Ethics declarations
Consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. The Committee for Biomedical Research of the Health Research Institute La Fe (Valencia, Spain) approved the protocol for involving the recruitment of the control group of healthy term infants (2019/0312) and the multicenter clinical trial, registered under the acronym HYPOTOP (EudraCT 2011–005696-17) [13]. Written informed consent was obtained from parents or legal representatives.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Daniele De Luca
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Máximo Vento is the senior author to this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cascant-Vilaplana, M.M., Piñeiro-Ramos, J.D., Soláz-García, Á. et al. Searching molecular biomarkers correlating with BSID-III at 24 months in infants with neonatal hypoxic-ischemic encephalopathy. Eur J Pediatr (2024). https://doi.org/10.1007/s00431-024-05652-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00431-024-05652-x