Abstract
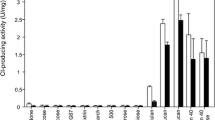
Pectin is one of the major cell wall polysaccharides found in dicotyledonous plants. We have solubilized and partially purified a β-(1→4)-galactosyltransferase (GalT) involved in the synthesis of the β-(1→4)-galactan side chains of pectin. The enzyme protein was almost completely solubilized by mixing a crude microsomal preparation of etiolated 6-day-old soybean (Glycine max Merr.) hypocotyls with a detergent, Triton X-100 (0.75%, w/v), in buffer. The solubilized enzyme was partially purified by ion-exchange chromatography. The crude membrane-bound GalT transferred Gal from UDP-Gal onto 2-aminobenzamide (AB)-derivatized β-(1→4)-galactoheptaose (Gal7-AB), leading to the formation of Gal8–11-AB by attachment of a series of one to four galactosyl residues; this is similar to what has previously been observed for 2-aminopyridine-derivatized β-(1→4)-galactooligomer acceptors (Konishi et al. in Planta 218:833–842, 2004). The partially purified GalT, by contrast, was able to transfer more than 25 galactosyl residues and elongated the chains to about Gal35-AB, thus almost reaching the length (43–47 Gal units) of native β-(1→4)-galactan side chains found in pectic polysaccharides from soybean cotyledons (Nakamura et al. in Biosci Biotechnol Biochem 66:1301–1313, 2002). Enzyme activity increased with increasing chain length of β-(1→4)-galactooligomers and reached maximal activity at heptaose, whereas galactooligomers higher than heptaose showed lower acceptor efficiency.





Similar content being viewed by others
Abbreviations
- AB:
-
2-Aminobenzamide
- DP:
-
Degree of polymerization
- GalA:
-
Galacturonic acid
- GalT:
-
Galactosyltransferase
- IMO:
-
Isomaltooligomer
- MALDI-TOF/MS:
-
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
- RG:
-
Rhamnogalacturonan
- l-Rha:
-
l-Rhamnose
References
Abdel-Massih RM, Baydoun EAH, Brett CT (2003) In vitro biosynthesis of 1,4-β-galactan attached to a pectin–xyloglucan complex in pea. Planta 216:502–511
Albersheim P, Nevins DJ, English PD, Karr A (1967) A method for the analysis of sugars in plant cell-wall polysaccharides by gas liquid chromatography. Carbohydr Res 5:340–345
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brickell LS, Reid JSG (1996) Biosynthesis in vitro of pectic (1→4)-β-d-galactan. In: Visser J, Voragen AGJ (eds) Pectins and pectinases. Elsevier, Amsterdam, pp 127–134
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Geshi N, Pauly M, Ulvskov P (2002) Solubilization of galactosyltransferase that synthesizes 1,4-β-galactan side chains in pectic rhamnogalacturonan I. Physiol Plant 114:540–548
Hakomori S (1964) A rapid permethylation of glycolipid, and polysaccharide catalyzed by methylsulfinyl carbanion in dimethyl sulfoxide. J Biochem 55:205–208
Harholt J, Jensen JK, Sørensen SO, Orfila C, Pauly M, Scheller HV (2006) ARABINAN DEFICIENT 1 is a putative arabinosyltransferase involved in biosynthesis of pectic arabinan in Arabidopsis. Plant Physiol 140:49–58
Ishii T, Ichita J, Matsue H, Ono H, Maeda I (2002) Fluorescent labeling of pectic oligosaccharides with 2-aminobenzamide and enzyme assay for pectin. Carbohydr Res 337:1023–1032
Ishii T, Ohnishi-Kameyama M, Ono H (2004) Identification of elongating β-1,4-galactosyltransferase activity in mung bean (Vigna radiata) hypocotyls using 2-aminobenzaminated 1,4-linked β-d-galactooligosaccharides as acceptor substrates. Planta 219:310–318
Ishikawa M, Kuroyama H, Takeuchi Y, Tsumuraya Y (2000) Characterization of pectin methyltransferase from soybean hypocotyls. Planta 210:782–791
Iwai H, Masaoka N, Ishii T, Satoh S (2002) A pectin glucuronyltransferase gene is essential for intercellular attachment in the plant meristem. Proc Natl Acad Sci USA 99:16319–16324
Keegstra K, Raikhel N (2001) Plant glycosyltransferases. Curr Opin Plant Biol 4:219–224
Konishi T, Mitome T, Hatsushika H, Haque MA, Kotake T, Tsumuraya Y. (2004) Biosynthesis of pectic galactan by membrane-bound galactosyltransferase from soybean (Glycine max Merr.) seedlings. Planta 218:833–842
McCartney L, Ormerod AP, Gidley MJ, Knox JP (2000) Temporal and spatial regulation of pectic (1→4)-β-d-galactan in cell walls of develo** pea cotyledons: implications for mechanical properties. Plant J 22:105–113
Nakamura A, Furuta H, Maeda H, Takao T, Nagamatsu Y (2002) Structural studies by stepwise enzymatic degradation of the main backbone of soybean soluble polysaccharides consisting of galacturonan and rhamnogalacturonan. Biosci Biotechnol Biochem 66:1301–1313
Nakano H, Takenishi S, Watanabe Y (1985) Purification and properties of two galactanases from Penicillium citrinum. Agric Biol Chem 49:3445–3454
Natsuka S, Adachi J, Kawaguchi M, Ichikawa A, Ikura K (2002) Method for purification of fluorescence-labeled oligosaccharides by pyridylamination. Biosci Biotechnol Biochem 66:1174–1175
Oberthür M, Peters S, Kumar Das S, Lichtenthaler FW (2002) Synthesis of linear β-(1→4)-galacto-hexa- and heptasaccharides and studies directed towards cyclogalactans. Carbohydr Res 337:2171–2180
O’Neill MA, York WS (2003) The composition and structure of plant primary cell walls. In: Rose JKC (ed) The plant cell wall. Annu Plant Reviews vol 8. Blackwell Publishing, Oxford, and CRC Press, Boca Raton, pp 1–54
Orfila C, Sørensen SO, Harholt J, Geshi N, Crombie H, Truong H-N, Reid JSG, Knox JP, Scheller HV (2005) QUASIMODO1 is expressed in vascular tissue of Arabidopsis thaliana inflorescence stems, and affects homogalacturonan and xylan biosynthesis. Planta 222:613–622
Peugnet I, Goubet F, Bruyant-Vannir MP, Thoiron B, Morvan C, Schols HA, Voragen AGJ (2001) Solubilization of rhamnogalacturonan I galactosyltransferases from membranes of a flax cell suspension. Planta 213:435–445
Ridley BL, O’Neill MA, Mohnen D (2001) Pectins: structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 57:929–967
Scheible W-R, Pauly M (2004) Glycosyltransferases and cell wall biosynthesis: novel players and insights. Curr Opin Plant Biol 7:285–295
Sterling JD, Atmodjo MA, Inwood SE, Kumar Kolli VS, Quigley HF, Hahn MG, Mohnen D (2006) Functional identification of an Arabidopsis pectin biosynthetic homogalacturonan galacturonosyltransferase. Proc Natl Acad Sci USA 103:5236–5241
Sørensen SO, Pauly M, Bush M, Skjøt M, McCann MC, Borkhardt B, Ulvskov P (2000) Pectin engineering: modification of potato pectin by in vivo expression of an endo-1,4-β-d-galactanase. Proc Natl Acad Sci USA 97:7639–7644
Acknowledgment
We thank Dr. H. Nakano, Osaka Municipal Research Institute, for the kind gift of endo-β-(1→4)-galactanase.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sugars described in this paper belong to the d-series unless otherwise noted.
Rights and permissions
About this article
Cite this article
Konishi, T., Kotake, T. & Tsumuraya, Y. Chain elongation of pectic β-(1→4)-galactan by a partially purified galactosyltransferase from soybean (Glycine max Merr.) hypocotyls. Planta 226, 571–579 (2007). https://doi.org/10.1007/s00425-007-0505-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-007-0505-3