Abstract
Purpose
Impaired gut barrier function is associated with systemic inflammation and many chronic diseases. Undigested dietary proteins are fermented in the colon by the gut microbiota which produces nitrogenous metabolites shown to reduce barrier function in vitro. With growing evidence of sex-based differences in gut microbiotas, we determined whether there were sex by dietary protein interactions which could differentially impact barrier function via microbiota modification.
Methods
Fermentation systems were inoculated with faeces from healthy males (n = 5) and females (n = 5) and supplemented with 0.9 g of non-hydrolysed proteins sourced from whey, fish, milk, soya, egg, pea, or mycoprotein. Microbial populations were quantified using fluorescence in situ hybridisation with flow cytometry. Metabolite concentrations were analysed using gas chromatography, solid phase microextraction coupled with gas chromatography-mass spectrometry and ELISA.
Results
Increased protein availability resulted in increased proteolytic Bacteroides spp (p < 0.01) and Clostridium coccoides (p < 0.01), along with increased phenol (p < 0.01), p-cresol (p < 0.01), indole (p = 0.018) and ammonia (p < 0.01), varying by protein type. Counts of Clostridium cluster IX (p = 0.03) and concentration of p-cresol (p = 0.025) increased in males, while females produced more ammonia (p = 0.02), irrespective of protein type. Further, we observed significant sex-protein interactions affecting bacterial populations and metabolites (p < 0.005).
Conclusions
Our findings suggest that protein fermentation by the gut microbiota in vitro is influenced by both protein source and the donor’s sex. Should these results be confirmed through human studies, they could have major implications for develo** dietary recommendations tailored by sex to prevent chronic illnesses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the exception of its role in food sensitivity and food allergy, dietary protein is seldom viewed as having anything other than positive effects on health. Consequently, dietary guidelines encouraging populations to increase protein intake by up to 100%, in a bid to combat the effects of sarcopenia [1], have been made with little consideration given to safety in terms of potential adverse effects on gut, immune and metabolic health. Until recently, digestion and absorption of dietary protein was considered to be highly efficient. However, recent evidence suggests that up to 10% of consumed protein reaches the colon undigested in humans [2]. The colon is host to the greatest density of commensal bacteria in mammals [3], and is where the highest level of bacterial fermentation of undigested food products occurs. Undigested colonic dietary protein is fermented by specific components of the resident microbiota [4] and thus has the potential to drive the expansion of proteolytic populations at the expense of more beneficial groups such as bifidobacteria and lactobacilli. Work in this area is limited, but intervention studies using varying amounts of increased dietary protein and time have reported changes in colonic bacterial metabolism [5,6,7], with increased dietary protein intake being linked to adverse shifts in bacterial populations [8]. Such changes can result in increases in the production of microbial-derived negative end-point metabolites, including ammonia and phenolic compounds, which have been shown to reduce gut barrier function in vitro [9,10,11,12]. The gut wall provides a physical, selective barrier preventing potentially harmful products of gut bacteria and food digestion from entering blood circulation [13]. Reductions in this barrier function through inflammation, disruption, and impaired integrity of the gut lining, a condition often referred to as ‘leaky gut’, can result in increased passage of these products, such as lipopolysaccharide (LPS), into blood [13, 14]. This can cause chronic low-grade systemic inflammation and thus promote the development of chronic degenerative diseases of the liver and cardiovascular system [14, 15]. While leaky gut may have multifactorial causes, fermentation of excess dietary protein by colonic microbes could be an important contributor to the development of these chronic diseases.
Healthy adult females have been shown to express lower and more variable permeability in the gut barrier than males. Additionally, adult male gut barrier function appears more stable and is less sensitive to impairment caused by non-steroidal anti-inflammatories (NSAIDs) than females [16]. However, female gut barrier function is more resilient to shock states such as acidosis or hypoxia than that of males [17], but becomes less stable with age [18]. The cause of these differences is multifactorial and is thought to involve sex hormones, immunity and the gut microbiota [19,20,21]. For example, female microbiotas are significantly more diverse than those in males, [22,23,24] and tend to have a lower abundance of Bacteroidetes, but higher proportions of Bacillota (Firmicutes) and Actinobacteria than male microbiotas [25, 26]. A greater diversity and high proportions of Bacillota can be indicative of a healthy microbiota [27], which might contribute to the greater efficiency observed in female barrier functionality. Microbiota sex differences also extend to lower taxonomic levels, with populations of Prevotellaceae, Ruminococcaceae [28], Clostridiales [22], and Escherichia [29] being lower in females than males. Specific microbial genera and species have been demonstrated to correlate with components of gastrointestinal immunity, including Clostridium-driven increases in intestinal mucosal regulatory T-cell (Treg) populations [30]. Higher levels of Clostridium populations in the male gut and the association between these microbes and increased levels of Tregs, may contribute to the disproportionally lower incidence of inflammatory gut conditions observed in males compared to females.
Complex interactions occur between gut barrier integrity, immune function and the gut microbiota. Since there is sexual dimorphism in all three of these systems in healthy adults, it is likely that the detrimental effects of excess dietary proteins on microbial-derived metabolites associated with reduced barrier function could also be sexually dimorphic. To the best of our knowledge, this has not yet been explored and possible mechanisms remain undefined. However, significant sex-dependent responses to dietary interventions have previously been identified, including inulin, starch and the probiotic Bifidobacteria lactis in immune-associated protein expression in the gut of 28-day-old piglets [31]. This supports potential links between nutrition and sex-based differences in physiological responses.
Consumption of proteins originating from different sources has previously been shown to have differential effects on metabolic end-product production by bacterial populations in the gut [32, 54,55,56].
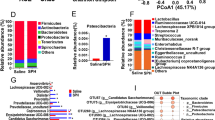
Increased protein availability was linked with significant shifts in the composition of the gut microbiota, with 7 out of the 11 functional groups quantified, differing significantly from the controls. Two of the most notable bacterial functional groups that were less prevalent under increased protein availability were Bifidobacterium and Lactobacillus spp genera. These groups include the most commonly used bacteria for probiotic supplementation, due to the large body of evidence that supports their positive effects on health [57,58,59], including reductions in intestinal permeability [60, 61]. In our trial, four phenolic metabolites (phenol, p-cresol, indole and ammonia), which have been shown to reduce barrier function in vitro [12], were significantly elevated under high protein conditions compared to the low protein conditions of the controls. These results are in accordance with a previously published study which also used in-vitro gut model systems [62]. However, most studies exploring the effects of high protein availability on microbiota composition and/or metabolic activity used hydrolysed protein in order to obtain high purity (∼ 95%), for example, casein hydrolysates [9, 62]. These ultra-concentrated sources of protein, limit levels of the non-protein substrates usually associated with these proteins when consumed as part of a normal human diet. These additional substrates may include non-digestible oligosaccharides, which are preferentially fermented by some gut bacteria before the proteins are utilised, and thus affect the composition of the gut microbiota. Therefore, using less pure proteins, as were used here (75–81%), is more reflective of normal high-protein diets and will generate results with higher translational potential than using ultra-pure protein substrates [9, 62]. Furthermore, hydrolysed proteins are absorbed absolutely in the upper intestinal tract [63]. Consequently, due to increased digestibility, it is highly unlikely that hydrolysed proteins reach the human colon where the majority of bacterial fermentation of dietary proteins occurs. This could, in part, explain the limited translation to in-vivo systems where increased dietary casein hydrolysate did not result in significant shifts in bacterial populations in human stools [9].
Although limited in number, the majority of published studies which explore the impact of increased dietary protein on microbiota composition and/or metabolic output do so by using protein derived from single sources [62, 9, 64,65,66]. However, here we demonstrate that bacterial fermentation of dietary proteins from different sources had differential impacts on colonic bacterial populations and metabolic end-products in vitro. The basis of this probably arises from disparities in the proteolytic capacity of different bacterial groups. For example, bacteria which secrete different proteases and peptidases, including specific species of Bacteroides, Clostridium and Fusobacterium [4, 67], have growth advantages over other bacterial groups in relation to availability of proteins from different sources. This is a result of varying capacities to cleave exogenous proteins into constituent amino acids, which are utilised more efficiently than larger peptides [68,69,70,71,72,73]. Subsequently, proteins from different sources are preferentially utilised by different proteolytic groups and thus differential population expansion would occur, as we observed. Linked to this, each of the tested proteins had unique amino acid compositions which may also provide growth advantages to bacterial groups that preferentially utilise specific amino acids [74, 75]. For example, when screened for amino acid content, Staphylococcus aureus had significantly higher levels of alanine than other Gram-positive bacteria. These bacteria can eithersynthesise alanine de novo, or have increased capacity to obtain alanine from extracellular sources [74]. Since all the proteins used in our trial were non-hydrolyzed, bacteria with lower proteolytic and higher saccharolytic capacity, such as Roseburia, were likely to have preferentially utilised any residual carbohydrates present in the protein additives [76]. This could explain why total numbers of Roseburia were significantly higher than controls in the non-animal proteins which are likely to contain additional fermentable carbohydrate compared to the animal proteins [4]. Since increased dietary protein is rarely a result of the consumption of an ultra-pure hydrolysed protein from a single source, our results better reflect the effects of high-protein diets on the composition and metabolic output of healthy human gut microbiotas.
Variation in amino acid compositions between the proteins assessed is highly likely to have contributed to the observed differential production of nitrogenous metabolites. The deamination of all three aromatic amino acids (phenylalanine, tyrosine and tryptophan) results in the production of phenol [77]. Similarly high phenylalanine and tryptophan content of milk, whey, soya and mycoprotein [78, 79] resulted in microbial fermentation of these proteins, producing significantly more phenol than fermentation of inulin. Furthermore, decarboxylation of tyrosine results in p-cresol production; since mycoprotein typically has low levels of tyrosine, it might be expected that it would produce less p-cresol than milk, fish and whey proteins which have relatively high levels of tyrosine [79]. However, we found that mycoprotein fermentation produced the highest concentration of p-cresol. While the reason for this is currently unclear, it could be due to the different changes in microbial composition between the proteins leading to elevated levels of p-cresol producers under mycoprotein conditions. Both phenol and p-cresol have been shown to increase permeability of colonocyte monolayers in vitro [21]. Taken together, our results demonstrate that the source of dietary protein should be taken into account when exploring the impact of high protein diets on microbiota composition and subsequent metabolic activity in relation to gut barrier functionality.
Our results are consistent with the growing body of evidence supporting sexual dimorphism in gut microbiotas [26, 80,81,82,83,84,85]. As previously noted, female gut microbiotas are often more diverse and have higher overall cell counts than male microbiotas [81, 85, 86]. However, the bacterial species reported to be different between the sexes is inconsistent. For example, males have been reported to have a greater abundance of the Bacteroides-Prevotella genera18,25,26, and certain species within the Bacteroides genus, than females [87], whilst, females have been found to have significantly more lactic acid producing bacteria than males [24]. Although we did observe higher overall bacterial cell counts in females, our study found no differences in the Bacteroidetes-Prevotellaceae (BAC) or Lactobacillus-Enterococcus (LAB) groups between the sexes at baseline. These discrepancies suggest there are as yet unknown caveats perhaps age could play a key role. Alternatively, our FISHflow methodology fully quantifies functional groups while deeper sequencing techniques may well have identified more subtle differences between the sexes at baseline. Additionally, reports of sex-based differences in gut microbiotas are often based on human trials where many additional influential factors are present. For example, bile acids [88] and components of immunity [89, 90], which have considerable impacts on gut microbiotas and are sexually dimorphic, but were absent from our in vitro models [91,92,93]. Regardless of this, we did observe significantly lower levels of Clostridium cluster IX in females than males, which is consistent with previous reports [81, 85, 86]. Interestingly, we report protein × sex interactions regarding changes in microbiota composition in response to increased fermentation of proteins from different sources. Here, Clostridium cluster IX (PRO) and Lactobacillus spp. (LAB) were present in greater numbers in males following fermentation of mycoprotein and whey protein than in females. Diet-dependent sex differences have previously been reported in animals and humans [82, 84, 94,95,96], with human males, in general, being more susceptible to dietary-driven changes in microbiota composition than females [82]. However, the underlying mechanisms have yet to be elucidated. During fermentation, differences in microbiotas may be exacerbated by dietary substrate availability, which could impact on bacterial metabolic activity. Although there were only limited sex-dependent microbiota compositional differences in response to fermentation of proteins from different sources, we did observe significant differences between the sexes in microbial metabolite production in response to differential dietary proteins. This is consistent with the metabolic potential of gut microbiotas varying between the sexes. This is of particular interest with regard to the microbial production of phenol in response to dietary fish protein, which was significantly higher in females compared to males. This finding is consistent with females being more susceptible to perturbations in gut barrier function in response to specific stimuli[17]. However, we also observed increases in propionate production by the gut microbiota in females in response to fish protein fermentation. Previously, propionate has been demonstrated to ameliorate dextran sodium sulphate-induced colitis in murine models by increasing expression of tight cell junction (TCJ)-associated proteins (e.g. Zona occludin, E-cadherin) [97]. Propionate has also been shown to promote expression of TCJ-associated proteins (claudins and occludins) in healthy pig jejunual mucosa [98].
In conclusion, our results describe strong correlations between increased dietary protein availability, shifts in microbial populations towards more proteolytic phenotypes and subsequent increases in bacterial metabolic end-products linked with increased intestinal permeability In vitro. Our results are consistent with our hypotheses and we demonstrate, for the first time, considerable sex-dependent influences on these interactions. Importantly, we show that both the source of protein is highly influential for microbiota composition and microbial metabolic outcomes. Perhaps caution should be exercised regarding blanket recommendations to increase protein consumption in the wake of the results reported here.
Data availability
The data that support the finding of this study are openly available in University of Reading Data Archive at DOI:https://doi.org/10.17864/1947.000504.
References
Stevenson EJ et al (2018) Protein for life: towards a focussed dietary framework for healthy ageing. Nutr Bull 43(1):97–102
Gilbert JA et al (2011) Effect of proteins from different sources on body composition. Nutr Metab Cardiovasc Dis 21(Suppl 2):B16–31
Hillman ET et al (2017) Microbial Ecology along the gastrointestinal tract. Microbes Environ 32(4):300–313
Smith EA, Macfarlane GT (1998) Enumeration of amino acid fermenting bacteria in the human large intestine: effects of pH and starch on peptide metabolism and dissimilation of amino acids. FEMS Microbiol Ecol 25(4):355–368
Beaumont M et al (2017) Quantity and source of dietary protein influence metabolite production by gut microbiota and rectal mucosa gene expression: a randomized, parallel, double-blind trial in overweight humans1. Am J Clin Nutr 106(4):1005–1019
Russell WR et al (2011) High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am J Clin Nutr 93(5):1062–1072
Geypens B et al (1997) Influence of dietary protein supplements on the formation of bacterial metabolites in the colon. Gut 41(1):70–76
McKenna CF et al (2021) Higher protein intake during resistance training does not potentiate strength, but modulates gut microbiota, in middle-aged adults: a randomized control trial. Am J Physiology-Endocrinology Metabolism 320(5):E900–E913
Beaumont M et al (2017) Quantity and source of dietary protein influence metabolite production by gut microbiota and rectal mucosa gene expression: a randomized, parallel, double-blind trial in overweight humans. Am J Clin Nutr 106(4):1005–1019
Hang I et al (2013) Impact of diets with a high content of greaves-meal protein or carbohydrates on faecal characteristics, volatile fatty acids and faecal calprotectin concentrations in healthy dogs. BMC Vet Res 9:201
McCall IC et al (2009) Effects of phenol on barrier function of a human intestinal epithelial cell line correlate with altered tight junction protein localization. Toxicol Appl Pharmacol 241(1):61–70
Yokoo K, Yamamoto Y, Suzuki T (2021) Ammonia impairs tight junction barriers by inducing mitochondrial dysfunction in Caco-2 cells. FASEB J 35(11):e21854
Vancamelbeke M, Vermeire S (2017) The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol 11(9):821–834
Miele L et al (2009) Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 49(6):1877–1887
Zhou X et al (2018) Gut-dependent microbial translocation induces inflammation and cardiovascular events after ST-elevation myocardial infarction. Microbiome 6(1):66
Edogawa S et al (2018) Sex differences in NSAID-induced perturbation of human intestinal barrier function and microbiota. FASEB J, fj201800560R
Homma H et al (2005) The female intestine is more resistant than the male intestine to gut injury and inflammation when subjected to conditions associated with shock states. Am J Physiol Gastrointest Liver Physiol 288(3):G466–G472
McOmber ME, Ou CN, Shulman RJ (2010) Effects of timing, sex, and age on site-specific gastrointestinal permeability testing in children and adults. J Pediatr Gastroenterol Nutr 50(3):269–275
Bruewer M et al (2003) Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol 171(11):6164–6172
van der Giessen J et al (2019) A direct effect of sex hormones on epithelial barrier function in inflammatory bowel Disease models. Cells 8(3):261
McCall IC et al (2009) Effects of phenol on barrier function of a human intestinal epithelial cell line correlate with altered tight junction protein localization. Toxicol Appl Pharmcol 241(1):61–70
Mahnic A, Rupnik M (2018) Different host factors are associated with patterns in bacterial and fungal gut microbiota in Slovenian healthy cohort. PLoS ONE 13(12):e0209209
Haro C et al (2016) Intestinal microbiota is influenced by gender and body Mass Index. PLoS ONE 11(5):e0154090
Borgo F et al (2018) Body Mass Index and Sex Affect Diverse Microbial niches within the gut. Front Microbiol, 9
Koliada A et al (2021) Sex differences in the phylum-level human gut microbiota composition. BMC Microbiol 21(1):131
Dominianni C et al (2015) Sex, body Mass Index, and Dietary Fiber Intake Influence the Human gut Microbiome. PLoS ONE 10(4):e0124599
Larsen OF, Claassen E (2018) The mechanistic link between health and gut microbiota diversity. Sci Rep 8(1):1–5
Oki K et al (2016) Comprehensive analysis of the fecal microbiota of healthy Japanese adults reveals a new bacterial lineage associated with a phenotype characterized by a high frequency of bowel movements and a lean body type. BMC Microbiol 16(1):284
Singh P, Manning SD (2016) Impact of age and sex on the composition and abundance of the intestinal microbiota in individuals with and without enteric infections. Ann Epidemiol 26(5):380–385
Atarashi K et al (2011) Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331(6015):337–341
Christoforidou Z et al (2019) Sexual dimorphism in Immune Development and in response to nutritional intervention in neonatal piglets. Front Immunol, 10
An C et al (2014) Caecal fermentation, putrefaction and microbiotas in rats fed milk casein, soy protein or fish meal. Appl Microbiol Biotechnol 98(6):2779–2787
**ao T et al (2021) Dietary proteins alter fermentation characteristics of human gut microbiota in Vitro. Plant Foods Hum Nutr 76(4):419–426
Bai G et al (2016) Dietary casein and soy protein isolate modulate the effects of Raffinose and Fructooligosaccharides on the composition and fermentation of gut microbiota in rats. J Food Sci, 81(8):H2093-8.
Nguyen TLA et al (2015) How informative is the mouse for human gut microbiota research? Dis Models Mech 8(1):1–16
**ao L et al (2015) A catalog of the mouse gut metagenome. Nat Biotechnol 33(10):1103–1108
Hugenholtz F, de Vos WM (2018) Mouse models for human intestinal microbiota research: a critical evaluation. Cell Mol Life Sci 75(1):149–160
Merrifield CA et al (2016) Neonatal environment exerts a sustained influence on the development of the intestinal microbiota and metabolic phenotype. ISME J 10(1):145–157
Schmidt B et al (2011) Establishment of normal gut microbiota is compromised under Excessive Hygiene conditions. PLoS ONE 6(12):e28284
Grimaldi R et al (2016) In vitro fermentation of B-GOS: impact on faecal bacterial populations and metabolic activity in autistic and non-autistic children. FEMS Microbiol Ecol, 93(2)
Richardson AJ et al (1989) Simultaneous determination of volatile and non-volatile acidic fermentation products of anaerobes by capillary gas chromatography. Lett Appl Microbiol 9(1):5–8
Rigottier-Gois L et al (2003) Fluorescent hybridisation combined with flow cytometry and hybridisation of total RNA to analyse the composition of microbial communities in human faeces using 16S rRNA probes FEMS Microbiol Ecol. 43(2):237–245
Rochet V et al (2004) Validation of fluorescent in situ hybridization combined with flow cytometry for assessing interindividual variation in the composition of human fecal microflora during long-term storage of samples. J Microbiol Methods 59(2):263–270
Wallner G, Amann R, Beisker W (1993) Optimizing fluorescent in situ hybridization with rRNA-targeted oligonucleotide probes for flow cytometric identification of microorganisms. Cytometry: J Int Soc Anal Cytol 14(2):136–143
Daims H et al (1999) The domain-specific probe EUB338 is Insufficient for the detection of all Bacteria: development and evaluation of a more comprehensive probe set. Syst Appl Microbiol 22(3):434–444
Langendijk PS et al (1995) Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in fecal samples. Appl Environ Microbiol 61(8):3069–3075
Harmsen HJM et al (2002) Extensive set of 16S rRNA-Based probes for detection of Bacteria in human feces. Appl Environ Microbiol 68(6):2982–2990
Manz W et al (1996) Application of a suite of 16S rRNA-specific oligonucleotide probes designed to investigate bacteria of the phylum cytophaga-flavobacter-bacteroides in the natural environment. Microbiology 142(5):1097–1106
Franks AH et al (1998) Variations of Bacterial Populations in Human Feces Measured by Fluorescent In Situ Hybridization with Group-Specific 16S rRNA-Targeted Oligonucleotide Probes Applied and Environmental Microbiology, 64(9):3336–3345
Walker AW et al (2005) pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within Microbial communities from the human Colon. Appl Environ Microbiol 71(7):3692–3700
Harmsen HJM et al (2000) Development of 16S rRNA-Based Probes for the < i > Coriobacterium Group and the < i > Atopobium Cluster and Their Application for Enumeration of < i > Coriobacteriaceae in Human Feces from Volunteers of Different Age Groups. Applied and Environmental Microbiology, 66(10): pp. 4523–4527
Hold GL et al (2003) Oligonucleotide Probes that detect quantitatively significant groups of butyrate-producing Bacteria in human feces. Appl Environ Microbiol 69(7):4320–4324
Devereux R et al (1992) Genus- and group-specific hybridization probes for determinative and environmental studies of sulfate-reducing Bacteria. Syst Appl Microbiol 15(4):601–609
Vulevic J et al (2015) Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. Br J Nutr 114(4):586–595
Liu Y, Gibson GR, Walton GE (2016) An in Vitro Approach to Study effects of Prebiotics and Probiotics on the faecal microbiota and selected Immune parameters relevant to the Elderly. PLoS ONE 11(9):e0162604
Walton GE et al (2012) A randomised crossover study investigating the effects of galacto-oligosaccharides on the faecal microbiota in men and women over 50 years of age. Br J Nutr 107(10):1466–1475
Wang Z et al (2022) The role of the gut microbiota and probiotics associated with microbial metabolisms in cancer prevention and therapy. Front Pharmacol, 13
Lewis MC et al (2013) Dietary supplementation with Bifidobacterium lactis NCC2818 from weaning reduces local immunoglobulin production in lymphoid-associated tissues but increases systemic antibodies in healthy neonates. Br J Nutr 110(7):1243–1252
Zhou J et al (2022) Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nat Commun 13(1):3432
Krumbeck JA et al (2018) Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome 6:1–16
Wang J et al (2018) Probiotic Lactobacillus plantarum promotes intestinal barrier function by strengthening the epithelium and modulating gut microbiota. Front Microbiol 9:1953
Wang X et al (2020) Prebiotics Inhibit Proteolysis by gut Bacteria in a host Diet-Dependent Manner: a three-stage continuous < i > in Vitro Gut Model Experiment. Appl Environ Microbiol 86(10):e02730–e02719
Koopman R et al (2009) Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am J Clin Nutr 90(1):106–115
Htoo JK et al (2007) Effect of dietary protein content on ileal amino acid digestibility, growth performance, and formation of microbial metabolites in ileal and cecal digesta of early-weaned pigs1,2. J Anim Sci 85(12):3303–3312
Opapeju FO et al (2009) Effect of dietary protein level on growth performance, indicators of enteric health, and gastrointestinal microbial ecology of weaned pigs induced with postweaning colibacillosis1,2. J Anim Sci 87(8):2635–2643
Hooda S et al (2013) The gut microbiome of kittens is affected by dietary protein:carbohydrate ratio and associated with blood metabolite and hormone concentrations. Br J Nutr 109(9):1637–1646
Allison C, Macfarlane GT (1989) Influence of pH, nutrient availability, and growth rate on amine production by Bacteroides fragilis and Clostridium perfringens. Appl Environ Microbiol 55(11):2894–2898
Nelson KE et al (2003) Complete genome sequence of the oral pathogenic bacterium < i > Porphyromonas gingivalis strain W83. J Bacteriol 185(18):5591–5601
Frandsen EV, Kjeldsen M, Kilian M (1997) Inhibition of Prevotella and Capnocytophaga immunoglobulin A1 proteases by human serum. Clin Diagn Lab Immunol 4(4):458–464
Yanagisawa M et al (2006) Proteinase Activity of Prevotella Species Associated with oral purulent infection. Curr Microbiol 52(5):375–378
Mills RH et al (2022) Multi-omics analyses of the ulcerative colitis gut microbiome link Bacteroides vulgatus proteases with disease severity. Nat Microbiol 7(2):262–276
McDermid AS, McKee AS, Marsh PD (1988) Effect of environmental pH on enzyme activity and growth of Bacteroides gingivalis W50. Infect Immun 56(5):1096–1100
Davila A-M et al (2013) Re-print of intestinal luminal nitrogen metabolism: role of the gut microbiota and consequences for the host. Pharmacol Res 69(1):114–126
Dai Z-L, Wu G, Zhu W-Y (2011) Amino acid metabolism in intestinal bacteria: links between gut ecology and host health. FBL 16(5):1768–1786
Barker HA (1981) Amino acid degradation by anaerobic bacteria. Annu Rev Biochem 50:23–40
Amaretti A et al (2019) Profiling of protein degraders in cultures of human gut microbiota. Front Microbiol 10:2614
Smith EA, Macfarlane GT (1997) Formation of Phenolic and Indolic compounds by anaerobic Bacteria in the human large intestine. Microb Ecol 33(3):180–188
Gorissen SHM et al (2018) Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 50(12):1685–1695
Coelho MOC et al (2019) Mycoprotein as a possible alternative source of dietary protein to support muscle and metabolic health. Nutr Rev 78(6):486–497
Mueller S et al (2006) Differences in fecal microbiota in different European study populations in relation to age, gender, and Country: a cross-sectional study. Appl Environ Microbiol 72(2):1027–1033
Yurkovetskiy L et al (2013) Gender bias in autoimmunity is influenced by microbiota. Immunity 39(2):400–412
Bolnick DI et al (2014) Individual diet has sex-dependent effects on vertebrate gut microbiota. Nat Commun 5(1):4500
Elderman M, de Vos P, Faas M (2018) Role of Microbiota in sexually dimorphic immunity. Front Immunol, 9(1018)
Org E et al (2016) Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 7(4):313–322
Sinha T et al (2019) Analysis of 1135 gut metagenomes identifies sex-specific resistome profiles. Gut Microbes 10(3):358–366
Valeri F, Endres K (2021) How biological sex of the host shapes its gut microbiota. Front Neuroendocr 61:100912
Li M et al (2008) Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci 105(6):2117–2122
Jia W, **e G, Jia W (2018) Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol 15(2):111–128
Sherman SB et al (2018) Prenatal androgen exposure causes hypertension and gut microbiota dysbiosis. Gut Microbes 9(5):400–421
Kim N (2022) Sex difference of gut microbiota Sex/gender-specific medicine in the gastrointestinal diseases, pp. 363–377
Phelps T et al (2019) The influence of biological sex and sex hormones on bile acid synthesis and cholesterol homeostasis. Biology sex Differences 10(1):1–12
Fisher M, Yousef I (1973) Sex differences in the bile acid composition of human bile: studies in patients with and without gallstones. Can Med Assoc J 109(3):190
Wu J et al (2020) Gender differences in the bile acid profiles of APP/PS1 transgenic AD mice. Brain Res Bull 161:116–126
Lim SM et al (2020) Sexually dimorphic response of increasing Dietary Intake of High Amylose Wheat on Metabolic and Reproductive outcomes in male and female mice. Nutrients 12(1):61
De Groef S et al (2022) Sexual dimorphism in metabolic responses to Western Diet in Drosophila melanogaster. Biomolecules 12(1):33
Mitchell SJ, Mitchell JR (2022) Sexual dimorphism in the response to dietary restriction in mice: A systematic review of the literature Nutrition and Healthy Aging, Preprint: pp. 1–34
Tong L-c et al (2016) Propionate ameliorates Dextran Sodium Sulfate-Induced colitis by improving intestinal barrier function and reducing inflammation and oxidative stress. Front Pharmacol, 7
Zhang Y et al (2019) Cecal infusion of Sodium Propionate promotes Intestinal Development and Jejunal Barrier function in growing pigs. Anim (Basel), 9(6)
Acknowledgements
The authors would like to acknowledge Food and Feed Innovations and Quorn for providing the proteins used in this study. We also acknowledge the BBSRC, FoodBiosystems DTP and Food and Feed Innovation for funding this research project. Of these funders, only Food and Feed Innovations made suggestions and recommendations towards the study design.
Funding
This work was primarily funded by the BBSRC under Grant BB/T008776/1 with additional funding from Food and Feed Innovations.
Author information
Authors and Affiliations
Contributions
M.C.L. and M.D.R designed the project and won the grant application. J.G. helped with the grant application and procured reagents used in the experiments. D.J. and C.P. conducted the in-vitro experiments. D.J. and B.L. conducted microbiota and short-chain fatty acid analysis. G.E.W. supported with the in-vitro experiments and provided insight on the interpretation of microbiota and metabolite analyses. J.S.E. conducted the phenolic compound analysis using SPME-GCMS and wrote the methods section for this specific measurement. D.J. and M.C.L. wrote the main manuscript text and prepared all figures. All authors reviewed the manuscript and provided feedback.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
One of the authors, John Gibson, works for Food and Feed Innovations which partly funded this research.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
James, D., Poveda, C., Walton, G.E. et al. Do high-protein diets have the potential to reduce gut barrier function in a sex-dependent manner?. Eur J Nutr (2024). https://doi.org/10.1007/s00394-024-03407-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00394-024-03407-w