Abstract
Objective
To measure the size of jugular foramina in infants affected by external hydrocephalus (EH) and in a control group, to support the hypothesis that a jugular foramen (JF) stenosis may determine dural venous sinus alterations and increased venous outflow resistance as main pathophysiological factor.
Methods
Minimum, maximum, and mean values of JF areas were measured in a series of phase-contrast magnetic resonance venous angiography (angio MRV PCA3D) performed on 81 infants affected by EH. Results were compared with a group of 54 controls.
Results
Smaller JF area was significantly smaller in patients versus controls (43.1 ± 14.6 vs. 52.7 ± 17.8; p < 0.001) resulting in a significantly smaller mean JF areas in patients vs. controls (51.6 ± 15.8 vs. 57.0 ± 18.3; p = 0.043). In patients, smaller JF areas were significantly associated with higher venous obstruction grading score (VOGS) both on the right (p = 0.018) and on the left side (p = 0.005). Positional plagiocephaly (cranial vault asymmetry index > 3.5%) was more frequent among EH patients than controls (38/17) but the difference was not significant (p = 0.07). In the 38 plagiocephalic patients, JF area was smaller on the flattened side than the contralateral in a significant number of cases both in right (21/7) and left (9/1) plagiocephaly (p < 0.0005) as well as the mean area (48.2 + 16.4 mm2 vs. 57.5 + 20.7 mm2, p = 0.002) and VOGS was significantly higher on the plagiocephalic side than on the contralateral side (1.6 ± 1.1 vs. 1.1 ± 0.9, p = 0.019).
Conclusion
In this series of infants affected by EH, the mean size of the ostium of both JF resulted significantly smaller than controls. JF stenosis was significantly associated with higher degrees of venous obstruction on both sides, suggesting a direct extrinsic effect of JF size on dural sinus lumen and possible consequent effect on venous outflow resistance. Positional plagiocephaly, when present, was associated with a decreased JF area and increased VOGS on the flattened side.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
External hydrocephalus is an infant condition that has been defined with many synonyms (external hydrocephalus [1,2,3,4,5], benign external hydrocephalus [6, 7], benign pericerebral collection, pericerebral hygromas [8], benign enlargement of the subarachnoid spaces (BESS) [9, 10], subarachnomegaly [11], familial macrocephaly [12], megalencephaly [13], macrocrania, pseudohydrocephalus-megalocephaly [14], chronic pericerebral effusions [15], and widened frontal subarachnoid spaces [16]). It is characterized by progressive macrocrania (head circumference > 98 percentile) usually diagnosed between 6 and 18 months of age, enlargement of subarachnoid spaces of the skull base and convexity, non-progressive ventricular dilatation [1, 11], axial hypotonia, delayed developmental milestones mainly depending upon gross motor delay that resolves in 86% of the cases within 22 months of age [8], high incidence (up to 8.1%) of subdural hematomas [9, 17], and more than double (up to 4.8%) incidence of autistic disorders when compared with normal population [6, 9]. Patients present a high incidence of dural venous sinus anomalies at the level of the posterior fossa affecting the sagittal sinus, both transverse sinuses and both sigmoid sinuses. These anomalies can be uni- or bilateral stenosis, uni- or bilateral flow gap, and/or uni- or bilateral agenesis. Combinations and severity of these strictures can be graded and the severity of the grading correlates proportionally with the amount of cerebrospinal fluid collection at the level of the convexities [1, 11]. The high incidence of these dural sinus anomalies compared to normal controls seems to confirm that external hydrocephalus in infants is associated with venous hypertension secondary to increased venous outflow resistance, according with the original theory of Portnoy and Croissant [13]. It remains to be defined why these patients are affected by intracranial venous hypertension. At least two similar pathophysiological models exist in the nature: achondroplasia and syndromic craniosynostosis of different types (Crouzon, Pfeiffer). In both conditions the original problem has been identified in the reduced size of the jugular foramen, induced by the hypoplasia of the chondrocranial part of the skull base in achondroplasia [18, 19] and by the early synostosis of the skull base synchondrosis including the petro-occipital synchondrosis that delimitates the jugular foramen in syndromic craniosynostosis [18, 20]. The dimensions of the jugular foramen of patients affected by infantile external hydrocephalus are not known. We report in this paper our analysis of this simple anatomical feature in a previously described cohort of selected patients and compare these data to similar measurements of a control group to verify the hypothesis that jugular foramen stenosis is present in infantile external hydrocephalus and could possibly be considered responsible of dural sinus stricture and consequent venous hypertension.
Methods
Patient population
Patients and controls were selected from a database of 97 infants affected by external hydrocephalus (EH) and 75 patients with normal head circumference used as control group that were prospectively collected between 2006 and 2020 at Santobono-Pausilipon Children’s Hospital, Naples, Italy. All these patients were studied with MR venous angiography (MRV). The neuroradiological findings of cerebrospinal (CSF) volumes of the ventricles and of the convexities, the presence of dural sinuses stenosis, and the correlations between CSF volumes and severity of stenosis have already been described elsewhere [1]. All the MR exams were reviewed for this study by two neuroradiologists (CR–AC) to verify that the sequences used for jugular foramen measurements were present and of adequate quality to allow manual measurement with pencil ROI method. Then, 16 patients and 21 controls did not meet the inclusion criteria because of movement artifacts or inadequate MR image quality and were excluded by the present study. The remaining 81 patients with EH and 54 controls were selected for the analysis and are the object of this report. Then, 24 of these 81 patients with EH also underwent a CT scan that were used for validation of jugular foramen asymmetry and of the cranial vault asymmetry index (CVAI).
MRI technique
Brain MRI was performed with a 1.5 T scanner (Ingenia Stream, Philips Medical Systems) without gadolinium administration. The patient was supine and received pharmacological sedation. Standardized protocol of brain MRI consisted by default of axial, sagittal, and coronal spin-echo T2-weighted imaging, axial spin-echo T1-weighted imaging, axial diffusion-weighted imaging (DWI) as well as 3D-PC-MRA. Additional sequences could be added upon clinical judgement by the responsible neuroradiologist. In 29 patients, volumetric T1 weighted sequences were performed.
Jugular foramen measurement on MRI
Sequences considered necessary to measure the jugular foramen area for the present study were sagittal 3D PC-MRA sequence (TR/TE 12/6 ms; field of view 230 × 230 mm, matrix size 256 × 192, slice thickness 1.6 mm, gap − 0.8 mm, flip angle 10°, and velocity-encoding factor for image acquisition 15 cm/sec). Post-processing of PC-MRA images for 3D reconstructions was performed by using the multiplanar (MPR) and the maximum intensity projection (MIP) reconstructions on a medical imaging viewing and processing software (HOROS Project, GNU Lesser General Public License, Version 3.0).
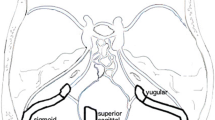
For each patient, the surface areas of right and left jugular foramina (JF) were measured using the pencil ROI method on axial MPR reconstructions (Fig. 1A, B) of the magnitude images from the PC-MRA sequence at the level of the skull base between the petrous temporal bone (laterally) and the jugular tubercle of the exocciput of the occipital bone (medially) according to Calandrelli et al. [21]. We considered smaller, larger, and mean values of JF areas for subsequent analysis. As the JF measurement method had been originally developed and validated for use on 3D-T1-weighted volumes [21], intra- and inter-rater reliability of JF measurements in the current experimental setup were preliminarily measured. To this end, on a subset of 74 studies, JF areas were measured twice 2 weeks apart by two neuroradiologists with at least 10 years of experience (CR and AC). Intra-class correlation coefficient (ICC) of the pooled right and left foramina measures was measured to assess intra-operator reliability. To measure intra-operator reproducibility, these 74 studies were also processed twice by one of the two neuroradiologists (CR). Jugular foramen areas were remeasured also on 29 MR exam including T1-weighted volumetric sequences (1-mm slice thickness) to validate the measure performed on the 3D PC-MRA sequences (1.6-mm thickness).
Plagiocephaly assessment
For each patient, the cranial vault asymmetry index (CVAI) was measured on the axial T2-weighted image closer to the superior orbital rim by drawing two 30° diagonals and dividing their difference by the length of the shorter diagonal [23, 24]. A cranial vault asymmetry index (CVAI) > 3.5% was considered diagnostic for positional plagiocephaly [22].
As CVAI has not been measured to date on MRI, we preliminarily validated the accuracy of this approach by comparing it with the same measure carried out on CT in 24 patients by correlation analysis.
Intracranial and CSF space measurements
Intracranial volume (ICV) and intracranial subarachnoid spaces volume (SASV) were calculated on axial T2-weighted images (TR/TE 6718 × 95 ms, FOV 220 × 220 mm, matrix size 284 × 213, slice thickness 2 mm, and flip angle 90°), as previously described [1]. Brush ROI method for segmentation of SASV and pencil ROI method for ICV segmentation as implemented in HOROS were used by 3 pediatric neuroradiologists (CR, AC, and FM).
The entity of widening of subarachnoid spaces was evaluated measuring the amount of cerebrospinal fluid of the convexity from the intercommissural plane until the last cut of the vertex where SAS were visible. Accordingly, ventricular volumes were calculated measuring the volume of the third and lateral ventricles from intercommissural plane to vertex. Intracranial volumes were calculated measuring the volume from the occipital foramen to vertex.
Venous obstruction grading score measurement
The presence of stenosis (scored 1), flow gap (scored 2), and agenesis (scored 3) was assessed for each transverse-sigmoid-jugular venous territory on 3D MIP reconstructions of PC-MRA sequences. Bilateral alterations were summed up providing for each patient a venous obstruction grading score (VOGS) ranging from 0 (normal venous drainage) through 6 (bilateral agenesis of both transverse-sigmoid axis) [1].
Statistical analysis
For each patient, the venous score, the smaller, larger, and mean of the left and right JF areas were considered for further statistical analysis as measures of the efficiency of the venous outflow.
Differences between EH patients and CTRL in the four venous outflow efficiency measures (smaller, larger, and mean of JF areas and venous grading) were tested on the whole dataset by stepwise binary logistic regression. In addition, the correlation of the three venous outflow efficiency measures with the head (ICV and HC) and subarachnoid space (SASV) were tested in the two groups separately by stepwise ordinal regression analysis. Correlation between jugular foramen size and VOGS was tested for each side independently both for patients and controls with Spearman correlation test. For all analyses, age and sex were entered in the model as nuisance covariates. Correlations with SASV were tested both with and without ICV in the model, to test effects also independent from overall head size. The effect of the presence of positional plagiocephaly on JF areas was tested by univariate analysis, including in the model age, sex, group, and side as nuisance covariates.
All statistical analyses were carried out using the Statistical Package for Social Science (SPSS) package (v.16.0, SPSS Inc., Chicago, USA), and p < 0.05 was considered significant, corrected for multiple comparisons according to Bonferroni where appropriate.
Results
All EH patients had by definition a head circumference above 98th percentile at the time of diagnosis. CTRL and EH patient groups were of comparable age at the time of angio MRV (15.2 ± 9.9 vs. 13.9 ± 8.6 months, respectively), while males were significantly more numerous in the EH group (63/81 vs. 26/54 in the CTRL group, p = 0.0004 at chi-squared test), in agreement with the general prevalence of EH in the European population [6]. Main clinical features of the two groups have already been described elsewhere [1]. The JF measurement method demonstrated excellent intra-operator (ICC = 0.942, range 0.920–0.958) and good inter-operator (ICC = 0.842, range 0.788–0.883) reproducibility, comparing favorably with the original procedure description (ICC = 0.72 and 0.91 for intra- and inter-rater reliability, respectively [21]). Measures of the JF obtained in the 29 patients whose exam contained volumetric T1-weighted sequences with 1-mm thick slices correlated well with the corresponding measures on 3D PC-MRA sequences with 1.6-mm thickness. In the 24 patients in whom a CT was available for comparison, CVAI measurements carried out on both scans showed an excellent correlation resulting in a Pearson’s correlation coefficient 0.955 (P < 10–12).
Right JF was larger in 31/54 (57.4%) of the controls and in 43/81 (53.1%) of the EH patients (n.s. at chi-squared test) as expected from physiological asymmetry [25]. Significantly higher asymmetry of the size of jugular foramen was observed instead in patients group.
In particular, the area of the smaller jugular foramen was significantly smaller in patients versus controls (43.1 ± 14.6 vs. 52.7 ± 17.8; p < 0.001 at Mann–Whitney U) (Fig. 2A) whereas no significant differences were observed in larger jugular foramen areas (60.0 ± 19.7 vs. 61.4 ± 19.5; p < 0.478) (Fig. 2B). This resulted in a significantly smaller mean jugular foramen area in patients vs controls (51.6 ± 15.8 vs. 57.0 ± 18.3; p = 0.043) (Fig. 2C).
A The smaller JF area of patients with EH are significantly smaller than the smaller JF area of controls. B The larger JF area of patient do not statistically differ from the larger JF area of controls. C The mean of JF areas of patients with EH is significantly smaller than the mean of JF areas of controls
In EH patients, VOGS was significantly higher compared to controls[1] (2.7 ± 1.6 vs. 0.7 ± 1.2; p < 10–11 at Mann–Whitney test) [1] and smaller jugular foramen areas were significantly associated with higher VOGS both on the right side (p = 0.018) (Fig. 3 up) and on the left side (p = 0.005) (Fig. 3 down).
This correlation was not significant in controls. Significance values at binary logistic regression analysis are shown in Table 1.
Fifty-five subjects had postural plagiocephaly (CVAI index > 3.5%) that was slightly more frequent among EH patients than controls (38/81 vs. 17/54) although this difference was not significant (p = 0.07) at chi-squared test. In the 38 EH patients with PP, 28 patients presented right side flattening and 10 patients presented left side flattening. In the 28 right plagiocephalic cases, JF area was smaller on the plagiocephalic side in 21 cases. In the 10 left plagiocephalic cases, JF area was smaller on the plagiocephalic side in 9 (p = 0.000066 at χ2 test). Overall, JF area was significantly smaller on the plagiocephalic side than on the contralateral side (48.2 + 16.4 vs. 57.5 ± 20.6, p = 0.002 at Wilcoxon signed rank test) (Fig. 4).
Left: Box and whisker plot comparing the jugular foramen areas of the 38 patients affected both by external hydrocephalus and positional plagiocephaly (CVAI > 3.5%). The jugular foramen area was significantly smaller on the flattened side (p = 0.002). Right: For each one of these 38 patients, the measure of the size of the jugular foramen on the flattened side was graphically linked (colored continued lines) to the contralateral measure in the same patient
In addition, VOGS was significantly higher (Fig. 5) on the plagiocephalic side than on the contralateral side (1.6 ± 1.1 vs 1.1 ± 0.9, p = 0.019 at Wilcoxon signed rank test) as expected [1] (Fig. 6).
Left: Box and whisker plot comparing the venous obstruction grading score (VOGS) of the 38 patients affected both by external hydrocephalus and positional plagiocephaly (CVAI > 3.5%). The VOGS was significantly higher on the flattened side (p = 0.019). Right: For each one of these 38 patients, the VOGS on the flattened side was graphically linked (colored continued lines) to the contralateral VOGS in the same patient
A Four-month-old boy evaluated for progressive macrocrania > 98° percentile and delayed milestones. CT scan shows external hydrocephalus, right positional plagiocephaly, and significant jugular foramen asymmetry (left > right). B At twelve months of age, MRI showed increased size of jugular foramen that remain asymmetric, confirm external hydrocephalus C with mild ventricular dilatation and right postural plagiocephaly (CVAI 10.75). D PC-MRV in coronal view confirms the different size of jugular foramen with consequent different caliber of jugular veins (left > right) and E, F 3D reconstruction of PC-MRV show a venous obstruction grading score (VOGS) of 4 due to bilateral flow gap at the level of jugular foramina
Discussion
The present study shows that the mean area of JF in a cohort of carefully selected external hydrocephalus patients is significantly smaller than a control group. Moreover, the physiological asymmetry between right and left foramen is significantly higher in external hydrocephalus (Figs. 4, 5, and 6). This data is even more significant when the smaller JF is considered. The dimension of the smallest JF remains significantly smaller in EH patients compared to control also when the presence of a higher VOGS is taken into account, thus indicating an additive effect of the obstruction at that level to the dural sinus stenosis. The significant association observed between postural plagiocephaly and jugular foramen stenosis (Fig. 7) is also of interest and add some elements to discussion on pathophysiology of EH.
Artistic drawing showing significant asymmetry in jugular foramen size, resulting in significant hypoplasia/stenosis of the homolateral transverse/sigmoid axis and development of significant collateral circulation as a consequence of increased VOGS. The resulting mean JF area will be significantly smaller than controls, inducing increased VrOut, consequent CSFROut finally resulting in external hydrocephalus
The theory of increased venous pressure
The pathophysiology of EH in infants remains matter of debate although the findings presented above suggest that the pathophysiology of EH could share some similitudes with that proposed for a similar condition observed in achondroplasia [19, 26,27,28,29].
Portnoy and Croissant in 1978 studied 7 patients affected by the condition with invasive CSF formation and absorption studies (CSFFA). They observed that all patients presented normal CSF absorption rate and high sagittal sinus pressure, concluding that “most likely, the defect is a high sagittal sinus pressure” [13]. During the CT scan era, the main advances in the knowledge of the condition have been the association with enlarged subarachnoid spaces of the cranial base and convexity in virtually all cases and the frequent association with a non-progressive ventricular dilatation in up to 80% of the cases [7] ranging from mild to severe depending on the series described [2, 3, 10, 12, 14,15,16, 30].
Angio MRV evidence of dural sinus stenosis
With the advent of MRI era came the awareness of the significant alterations in the anatomy of the dural sinuses of the posterior fossa under the form of stenosis, flow gap or complete agenesis [4]. The degree of alterations calculated with purely morphological VOGS correlated well with the amount of CSF measured in the subarachnoid spaces using two different scores [1, 5] and, therefore, had to be considered as real radiological manifestation of impaired venous circulation at the level of the main dural sinuses of the posterior fossa. These alterations could be responsible for the increased venous pressure postulated by Portnoy and Croissant as main pathophysiological factor of EH. Bateman and Napier showed in 2011 that in patients with EH the rate of arterial blood flow drained through the sagittal sinus is significantly lower than that observed in healthy controls (37% vs. 52%), offering further evidence in favor of the existence of an increased venous outflow resistance (VROut) with increased upstream capillary bed pressure in patients with EH [4]. On these bases, they postulated that CSF resorption was impaired at the level of the capillary bed, where resorption is supposed to occur in this age range due to the lack or scarcity of Pacchioni granulations in this age range [31].
Jugular foramen stenosis
Stenosis of the jugular foramen with consequent stricture of the sigmoid sinus where it enters the jugular bulb in fact may critically interfere with the rapid ballooning of the jugular sinus (from 1 mm at birth to 5 mm at 12 months of age) that typically occurs in this age range [26], creating the anatomical condition to increase the VROut and explain the increased sagittal sinus pressure observed in EH [13]. Nevertheless, in spite of the appealing findings, the real pathophysiological mechanism of external hydrocephalus in infants is certainly more complex and should take into account the cascade of events that takes place in the intracranial cavity of an infant from birth until 2 years of age (Fig. 7A–D). In fact, brain weight quadruples in the first three years of life going from 0.36 to 0.38 kg (F/M) at birth to 1.09–1.27 kg (F/M) between 31 and 43 months [32]. Therefore, global cerebral blood flow increases following a linear correlation between age and weight in the first year of life [33, 34] even if brain perfusion remains relatively stable around 25 mL/min/kg body weight. This increase in blood flow impacts against the significantly smaller mean JF area with consequent uni-/bilateral reduced dural sinus volume, resulting in increased VROut with dilatation of cortical veins and of the sagittal sinus (arrow 5), increased capillary bed resistance, and consequent increased CSFROut due to altered CSF resorption at the level of the capillary bed (Fig. 8) [26, 26, 35]. The significantly smaller mean of jugular foramen size in patients with external hydrocephalus, if confirmed by further CT and MR studies, seems to be only a very small element but can play a very strategic role in this pathophysiological cascade of events that is quite well accepted and recognized in achondroplasia [19, 29] and syndromic craniosynostosis[18].
Artistic representation of the dynamic changes probably occurring in the first 2 years of life in external hydrocephalus. Beyond the certain anatomical data illustrated (reduced mean JF size, dural sinus stenosis, increased CSF spaces and ventricular size), the succession of events remains speculative. A EH patients are typically born with normal head circumference (HC). In the first 3–4 months after birth, jugular stenosis is probably already present but the two stenotic jugular foramens still allow adequate venous outflow through it (arrow 1). Persistent dural sinus lakes on the midline (arrow 2) contribute to venous drainage. Arachnoid villi are still under-represented and bridging veins have normal size (arrow 3). This steady state equilibrium does not compromise the capillary bed pressure and allows adequate resorption of the CSF through the capillary bed without increasing the CSF outflow resistance (CSFROut), thus maintaining a normal CSF resorption rate with little or no CSF accumulation in the subarachnoid spaces (arrow 4) and normal head growth below 95th percentile. B Rapidly increasing blood flow through the rapidly growing brain that normally induces the very rapid ballooning of the jugular bulb with formation of the sigmoid-jugular transition at the level of the jugular foramen becomes hemodynamically evident also in the sagittal sinus around 4–6 months of age (arrow 5). This impacts against the significantly smaller mean JF area that does not allow this critical step with consequent uni/bilateral reduced dural sinus volume, that associated with physiological involution of midline venous lakes (arrow 6) results in increased VROut with dilatation of cortical veins and of the sagittal sinus (arrow 5), increased capillary bed resistance, and consequent increased CSFROut due to altered CSF resorption at the level of the capillary bed. This leads to accumulation of CSF in the subarachnoid spaces and in the ventricles (arrow 8) with abnormal head growth between the 4th and 6th month and crosses the 97th percentile around the 6th month of age, allowing diagnosis by ultrasound or CT scan between 6 and 12th month. C The CSF dynamics remain unbalanced. CSF accumulation in the subarachnoid spaces and in the cerebral ventricles (arrow 9) determines rapidly progressive macrocrania with head circumference crossing the 98th percentile between 5 and 7th month of age, continuing to increase until the first year of life. Venous collaterals begin to develop throughout the neurocranium contributing to stabilize the VROut (arrow 10). D Between the 12th and 18th months of life several events occur that allow slow adaptation to this challenging condition that typically finds a steady state around the end of the second year of life maintaining stable pericerebral CSF spaces and ventricles (arrow 11): sudden stabilization of sagittal sinus blood flow, that after steep increase from < 200 mLs/min at 6 months to > 450 mLs/min around 18 months stabilizes on values very close to the blood flow (500 mLs/min) of older children until 6 years of age [35]. Transverse sinuses and upper jugular bulb tend to enlarge favouring formation of collateral peri-jugular venous circulation through the anterior and posterior condylar veins, mastoid emissary veins and occipital emissary vein [26] (arrow 12). Arachnoid villi progressively increase in number and size [36]. The role of this factor remains to be determined since this modification has no direct effect on RVOut, but the progressive formation of arachnoid villi, increasing the resorption sites and modality, may lower the CSFROut, thus contributing to reach a steady state in CSF hydrodynamics and regularizing the speed of growth of head circumference
Postural plagiocephaly
This study shows that a cranial vault asymmetry index (CVAI) > 3.5% normally referred as positional plagiocephaly [22], whatever its origin, contributes to the pathophysiology of the condition determining the side of the smaller JF that is significantly associated with an increased VOGS on the flattened side. The possible role of the vector of deformation of the occipital squama on the petro-occipital synchondrosis with narrowing of the JF and, possibly, on the lumen of the transverse and sigmoid sinus, remains matter of speculation but the strong correlation found in our case series warrants further studies.
Limitations of this study are the lack of CT scan confirmation of the measures of the jugular foramen area, the fact that only the inlet area of the jugular foramen was measured not kee** into account the anatomical complexity of the jugular foramen nor the outlet area, the numeric difference between the patients group and the control group due to the difficulty of finding normal MRI exams with angio MR venography in this age range. Further studies are warranted in the genetic field to verify the integrity of FGFR genes and ascertain the possible similitude of the pathophysiology of external hydrocephalus with the abovementioned diseases.
Conclusions
In this case series of patients with external hydrocephalus, the size of the smaller jugular foramen and the mean size of both jugular foramens resulted significantly smaller than the control group. This anatomical detail adds further evidence in favor of the increased venous outflow resistance (VROut) as main factor in the pathophysiology of external hydrocephalus in infants.
Figures 7 and 8 have been produced thanks to the generous financial contribution from “Fondazione Santobono-Pausilipon” (https://fondazionesantobonopausilipon.it/).
Data availability
The data presented and analyzed in this study are available upon request from the corresponding author. The data are not publicly available due to privacy restrictions.
References
Cinalli G, di Martino G, Russo C et al (2021) Dural venous sinus anatomy in children with external hydrocephalus: analysis of a series of 97 patients. Childs Nerv Syst 37:3021–3032. https://doi.org/10.1007/s00381-021-05322-5
Maytal J, Alvarez L, Elkin C, Shinnar S (1987) External hydrocephalus: radiologic spectrum and differentiation from cerebral atrophy. Am J Roentgenol 148:1223–1230. https://doi.org/10.2214/ajr.148.6.1223
Barlow CF (1984) CSF dynamics in hydrocephalus—with special attention to external hydrocephalus. Brain Dev 6:119–127. https://doi.org/10.1016/S0387-7604(84)80060-1
Bateman GA, Napier BD (2011) External hydrocephalus in infants: six cases with MR venogram and flow quantification correlation. Childs Nerv Syst 27:2087–2096. https://doi.org/10.1007/s00381-011-1549-z
Sainz LV, Zipfel J, Kerscher SR et al (2019) Cerebro-venous hypertension: a frequent cause of so-called “external hydrocephalus” in infants. Childs Nerv Syst 35:251–256. https://doi.org/10.1007/s00381-018-4007-3
Zahl SM, Egge A, Helseth E, Wester K (2019) Clinical, radiological, and demographic details of benign external hydrocephalus: a population-based study. Pediatr Neurol 96:53–57. https://doi.org/10.1016/j.pediatrneurol.2019.01.015
Wiig US, Zahl SM, Egge A et al (2017) Epidemiology of benign external hydrocephalus in Norway—a population-based study. Pediatr Neurol 73:36–41. https://doi.org/10.1016/j.pediatrneurol.2017.04.018
Maruccia F, Gomáriz L, Rosas K et al (2021) Neurodevelopmental profile in children with benign external hydrocephalus syndrome. A pilot cohort study. Childs Nerv Syst 37:2799–2806. https://doi.org/10.1007/s00381-021-05201-z
Holste KG, Wieland CM, Ibrahim M et al (2022) Subdural hematoma prevalence and long-term developmental outcomes in patients with benign expansion of the subarachnoid spaces. J Neurosurg Pediatr 29:536–542. https://doi.org/10.3171/2021.12.PEDS21436
Ment LR, Duncan CC, Geehr R (1981) Benign enlargement of the subarachnoid spaces in the infant. J Neurosurg 54:504–508. https://doi.org/10.3171/jns.1981.54.4.0504
Sainz LV, Schuhmann MU (2021) Subarachnomegaly—venous congestion of infancy. Childs Nerv Syst 37:3455–3463. https://doi.org/10.1007/s00381-021-05328-z
Alvarez LA, Maytal J, Shinnar S (1986) Idiopathic external hydrocephalus: natural history and relationship to benign familial macrocephaly. Pediatrics 77:901–907
Portnoy HD (1978) Megalencephaly in infants and children. Arch Neurol 35:306. https://doi.org/10.1001/archneur.1978.00500290052009
Sahar A (1978) Pseudohydrocephalus-megalocephaly, increased intracranial pressure and widened subarachnoid space. Neuropediatrics 9:131–139. https://doi.org/10.1055/s-0028-1085418
Baraton J, Brunelle F, Pierre-Kahn A et al (1989) X-ray computed tomography coupled with cisternography in chronic pericerebral effusions in young children. Neurochirurgie 35(395–400):411
Odita JC (1992) The widened frontal subarachnoid space. Child’s Nervous System 8:36–39. https://doi.org/10.1007/BF00316560
Andersson J, Wikström J, Högberg U et al (2022) External hydrocephalus as a cause of infant subdural hematoma: epidemiological and radiological investigations of infants suspected of being abused. Pediatr Neurol 126:26–34. https://doi.org/10.1016/j.pediatrneurol.2021.09.018
Sainte-Rose C, LaCombe J, Pierre-Kahn A et al (1984) Intracranial venous sinus hypertension: cause or consequence of hydrocephalus in infants? J Neurosurg 60:727–736. https://doi.org/10.3171/jns.1984.60.4.0727
Pierre-Kahn A, Hirsch JF, Renier D et al (1980) Hydrocephalus and achondroplasia. A study of 25 observations. Childs Brain 7:205–219
Florisson JMG, Barmpalios G, Lequin M et al (2015) Venous hypertension in syndromic and complex craniosynostosis: the abnormal anatomy of the jugular foramen and collaterals. J Craniomaxillofac Surg 43:312–318. https://doi.org/10.1016/j.jcms.2014.11.023
Calandrelli R, Panfili M, D’Apolito G et al (2017) Quantitative approach to the posterior cranial fossa and craniocervical junction in asymptomatic children with achondroplasia. Neuroradiology 59:1031–1041. https://doi.org/10.1007/s00234-017-1887-y
Loveday BP, de Chalain TB (2001) Active counterpositioning or orthotic device to treat positional plagiocephaly? J Craniofac Surg 12:308–313. https://doi.org/10.1097/00001665-200107000-00003
Calandrelli R, Pilato F, Massimi L et al (2024) Computed tomography quantitative analysis of cranial vault dysmorphology and severity of facial complex changes in posterior synostotic plagiocephaly patients. Childs Nerv Syst 40:779–790. https://doi.org/10.1007/s00381-023-06227-1
Miyabayashi H, Saito K, Kato R et al (2023) Denominator of cranial vault asymmetry index: choosing between longer and shorter diagonal lengths. J Craniofac Surg 34:e369–e372. https://doi.org/10.1097/SCS.0000000000009263
de Freitas CAF, dos Santos LRM, Santos AN et al (2020) Anatomical study of jugular foramen in the neck. Braz J Otorhinolaryngol 86:44–48. https://doi.org/10.1016/j.bjorl.2018.09.004
Okudera T, Huang YP, Ohta T et al (1994) Development of posterior fossa dural sinuses, emissary veins, and jugular bulb: morphological and radiologic study. AJNR Am J Neuroradiol 15:1871–1883
Shim Y, Ko JM, Cho T-J et al (2021) Predictors of cervical myelopathy and hydrocephalus in young children with achondroplasia. Orphanet J Rare Dis 16:81. https://doi.org/10.1186/s13023-021-01725-4
Mukherjee D, Pressman BD, Krakow D et al (2014) Dynamic cervicomedullary cord compression and alterations in cerebrospinal fluid dynamics in children with achondroplasia: review of an 11-year surgical case series. J Neurosurg Pediatr 14:238–244. https://doi.org/10.3171/2014.5.PEDS12614
Bosemani T, Orman G, Hergan B et al (2015) Achondroplasia in children: correlation of ventriculomegaly, size of foramen magnum and jugular foramina, and emissary vein enlargement. Childs Nerv Syst 31:129–133. https://doi.org/10.1007/s00381-014-2559-4
Kendall B, Holland I (1981) Benign communicating hydrocephalus in children. Neuroradiology 21:93–96. https://doi.org/10.1007/BF00342987
Oi S, Di Rocco C (2006) Proposal of “evolution theory in cerebrospinal fluid dynamics” and minor pathway hydrocephalus in develo** immature brain. Childs Nerv Syst 22:662–669. https://doi.org/10.1007/s00381-005-0020-4
Dekaban AS, Sadowsky D (1978) Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights. Ann Neurol 4:345–356. https://doi.org/10.1002/ana.410040410
Benders MJNL, Hendrikse J, de Vries LS et al (2011) Phase-contrast magnetic resonance angiography measurements of global cerebral blood flow in the neonate. Pediatr Res 69:544–547. https://doi.org/10.1203/PDR.0b013e3182176aab
Kehrer M, Goelz R, Krägeloh-Mann I, Schöning M (2002) Measurement of volume of cerebral blood flow in healthy preterm and term neonates with ultrasound. Lancet 360:1749–1750. https://doi.org/10.1016/S0140-6736(02)11720-X
Hirabuki N, Watanabe Y, Mano T et al (2000) Quantitation of flow in the superior sagittal sinus performed with cine phase-contrast MR imaging of healthy and achondroplastic children. AJNR Am J Neuroradiol 21:1497–1501
Gomez DG, Ehrmann JE, Potts DG et al (1983) The arachnoid granulations of the newborn human: an ultrastructural study. Int J Dev Neurosci 1:139–145. https://doi.org/10.1016/0736-5748(83)90040-0
Acknowledgements
Dr Mario Quarantelli participates in the present work upon official deliberation n. 197 of September 28th, 2020 of Santobono-Pausilipon Children’s Hospital (AORN) certifying the cooperation agreement between Biostructure and Bioimaging Institute, National Research Council, Naples, Italy and Santobono-Pausilipon Children’s Hospital (AORN), Naples, Italy. We thank Cura Canaz Medical Arts (www.ccmedicalarts.com) for the illustrations.
Author information
Authors and Affiliations
Contributions
GC had the original research idea, wrote the main manuscript, and overviewed the work of measuring. GDM collected the clinical data. CR, AC, and FM measured the jugular foramens and gave the venous obstruction grading score for each patient. SP contributed to statistical analysis. MAC reviewed the manuscript and references and prepared and formatted the figures. GM and PS contributed to collect clinical data. MQ did the statistics. EC overviewed and coordinated radiological evaluations.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cinalli, G., Di Martino, G., Russo, C. et al. Jugular foramen stenosis in external hydrocephalus in infants. Childs Nerv Syst 40, 2081–2091 (2024). https://doi.org/10.1007/s00381-024-06414-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-024-06414-8
Keywords
- External hydrocephalus
- Jugular foramen
- Benign pericerebral collection
- Benign external hydrocephalus
- Benign enlargement of the subarachnoid spaces–BESS
- Subarachnomegaly
- Macrocephaly
- Macrocrania
- Venous obstruction grading score
- Venous hypertension
- Postural plagiocephaly
- Positional plagiocephaly
- Hydrocephalus
- Infants