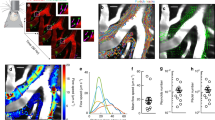
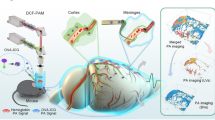
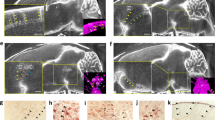
Abstract
Over the past decade, there has been a tremendously increased interest in understanding the neurophysiology of cerebrospinal fluid (CSF) flow, which plays a crucial role in clearing metabolic waste from the brain. This growing interest was largely initiated by two significant discoveries: the glymphatic system (a pathway for solute exchange between interstitial fluid deep within the brain and the CSF surrounding the brain) and meningeal lymphatic vessels (lymphatic vessels in the layer of tissue surrounding the brain that drains CSF). These two CSF systems work in unison, and their disruption has been implicated in several neurological disorders including Alzheimer’s disease, stroke, and traumatic brain injury. Here, we present experimental techniques for in vivo quantification of CSF flow via direct imaging of fluorescent microspheres injected into the CSF. We discuss detailed image processing methods, including registration and masking of stagnant particles, to improve the quality of measurements. We provide guidance for quantifying CSF flow through particle tracking and offer tips for optimizing the process. Additionally, we describe techniques for measuring changes in arterial diameter, which is an hypothesized CSF pum** mechanism. Finally, we outline how these same techniques can be applied to cervical lymphatic vessels, which collect fluid downstream from meningeal lymphatic vessels. We anticipate that these fluid mechanical techniques will prove valuable for future quantitative studies aimed at understanding mechanisms of CSF transport and disruption, as well as for other complex biophysical systems.






Similar content being viewed by others
Data Availability
A short working example including two-photon images and MATLAB scripts is available at https://doi.org/10.5281/zenodo.8165799.
References
Abbott NJ (2004) Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int 45(4):545–552
Abbott NJ, Pizzo ME, Preston JE, Janigro D, Thorne RG (2018) The role of brain barriers in fluid movement in the CNS: is there a ‘glymphatic’ system? Acta Neuropathol 135(3):387–407
Agarwal N, Carare RO (2021) Cerebral vessels: an overview of anatomy, physiology, and role in the drainage of fluids and solutes. Front Neurol 11:1748
Ahn JH, Cho H, Kim J-H, Kim SH, Ham J-S, Park I, Suh SH, Hong SP, Song J-H, Hong Y-K, Jeong Y, Park S-H, Koh GY (2019) Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 572(7767):62–66. https://doi.org/10.1038/s41586-019-1419-5
Albargothy NJ, Johnston DA, MacGregor-Sharp M, Weller RO, Verma A, Hawkes CA, Carare RO (2018) Convective influx/glymphatic system: tracers injected into the CSF enter and leave the brain along separate periarterial basement membrane pathways. Acta Neuropathol 136(1):139–152
Aldea R, Weller RO, Wilcock DM, Carare RO, Richardson G (2019) Cerebrovascular smooth muscle cells as the drivers of intramural periarterial drainage of the brain. Front Aging Neurosci 11:1
Arnold W, Ritter R, Wagner W (1973) Quantitative studies on the drainage of the cerebrospinal fluid into the lymphatic system. Acta Oto-Laryngol 76(1–6):156–161
Asgari M, Zélicourt D, Kurtcuoglu V (2016) Glymphatic solute transport does not require bulk flow. Sci Rep 6(1):38635
Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K (2015) A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 212(7):991–999
Battal B, Kocaoglu M, Bulakbasi N, Husmen G, Tuba Sanal H, Tayfun C (2011) Cerebrospinal fluid flow imaging by using phase-contrast MR technique. Brit J Radiol 84(1004):758–765
Bedussi B, Almasian M, Vos J, VanBavel E, Bakker EN (2018) Paravascular spaces at the brain surface: Low resistance pathways for cerebrospinal fluid flow. J Cerebr Blood F Met 38(4):719–726
Benveniste H, Liu X, Koundal S, Sanggaard S, Lee H, Wardlaw J (2019) The glymphatic system and waste clearance with brain aging: a review. Gerontology 65(2):106–119
Bohr T, Hjorth PG, Holst SC, Hrabětová S, Kiviniemi V, Lilius T, Lundgaard I, Mardal K-A, Martens EA, Mori Y, Nägerl UV, Nicholson C, Tannenbaum A, Thomas JH, Tithof J, Benveniste H, Iliff JJ, Kelley DH, Nedergaard M (2022) The glymphatic system: Current understanding and modeling. iScience 25(9):104987. https://doi.org/10.1016/j.isci.2022.104987
Bojarskaite L, Vallet A, Bjørnstad DM, Gullestad Binder KM, Cunen C, Heuser K, Kuchta M, Mardal K-A, Enger R (2023) Sleep cycle-dependent vascular dynamics in male mice and the predicted effects on perivascular cerebrospinal fluid flow and solute transport. Nat Commun 14(1):953
Boster KAS, Tithof J, Cook DD, Thomas JH, Kelley DH (2022) Sensitivity analysis on a network model of glymphatic flow. J Roy Soc Interface. https://doi.org/10.1098/rsif.2022.0257
Bradbury M, Cole D (1980) The role of the lymphatic system in drainage of cerebrospinal fluid and aqueous humour. J Physiol 299(1):353–365
Bradley W (2015) CSF flow in the brain in the context of normal pressure hydrocephalus. Am J Neuroradiol 36(5):831–838
Bèchet NB, Shanbhag NC, Lundgaard I (2021) Glymphatic pathways in the gyrencephalic brain. J Cerebr Blood F Met 41(9):2264–2279
Carare R, Bernardes-Silva M, Newman T, Page A, Nicoll J, Perry V, Weller R (2008) Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropath Appl Neuro 34(2):131–144
Carr JB, Thomas JH, Liu J, Shang JK (2021) Peristaltic pum** in thin non-axisymmetric annular tubes. J Fluid Mech. https://doi.org/10.1017/jfm.2021.277
Charogiannis A, An JS, Markides CN (2015) A simultaneous planar laser-induced fluorescence, particle image velocimetry and particle tracking velocimetry technique for the investigation of thin liquid-film flows. Exp Therm Fluid Sci 68:516–536
Cheng KP, Brodnick SK, Blanz SL, Zeng W, Kegel J, Pisaniello JA, Ness JP, Ross E, Nicolai EN, Settell ML et al (2020) Clinically-derived vagus nerve stimulation enhances cerebrospinal fluid penetrance. Brain Stimul 13(4):1024–1030. https://doi.org/10.1016/j.brs.2020.03.012
Choi S, Jang DC, Chung G, Kim SK (2022) Transcutaneous auricular vagus nerve stimulation enhances cerebrospinal fluid circulation and restores cognitive function in the rodent model of vascular cognitive impairment. Cells 11(19):3019
Croci M, Vinje V, Rognes ME (2019) Uncertainty quantification of parenchymal tracer distribution using random diffusion and convective velocity fields. Fluids Barriers CNS 16(1):1–21
Cserr HF, Cooper DN, Suri PK, Patlak CS (1981) Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am J Physiol–Renal 240(4):319–328
Cserr HF, Ostrach L (1974) Bulk flow of interstitial fluid after intracranial injection of blue dextran 2000. Exp Neurol 45(1):50–60
Decker Y, Krämer J, **n L, Müller A, Scheller A, Fassbender K, Proulx ST (2022) Magnetic resonance imaging of cerebrospinal fluid outflow after low-rate lateral ventricle infusion in mice. JCI Insight 7(3):e150881
Diem AK, MacGregor Sharp M, Gatherer M, Bressloff NW, Carare RO, Richardson G (2017) Arterial pulsations cannot drive intramural periarterial drainage: significance for a \(\beta\) drainage. Front Neurosci 11:475
Diezmann L, Shechtman Y, Moerner W (2017) Three-dimensional localization of single molecules for super-resolution imaging and single-particle tracking. Chem Rev 117(11):7244–7275
Dixon JB, Zawieja DC, Gashev AA, Coté GL (2005) Measuring microlymphatic flow using fast video microscopy. J Biomed Opt 10(6):064016–064016
Du T, Mestre H, Kress BT, Liu G, Sweeney AM, Samson AJ, Rasmussen MK, Mortensen KN, Bork PAR, Peng W, Olveda GE, Bashford L, Toro ER, Tithof J, Kelley DH, Thomas JH, Hjorth PG, Martens EA, Mehta RI, Hirase H, Mori Y, Nedergaard M (2021) Cerebrospinal fluid is a significant fluid source for anoxic cerebral oedema. Brain 145(2):787–797. https://doi.org/10.1093/brain/awab293
Du T, Raghunandan A, Mestre H, Plá V, Liu G, Ladrón-de-Guevara A, Nedergaard M, Kelley DH (2023). Restoration of cervical lymphatic vessel function in aging rescues cerebrospinal fluid drainage. Submitted
Eide PK, Vatnehol SAS, Emblem KE, Ringstad G (2018) Magnetic resonance imaging provides evidence of glymphatic drainage from human brain to cervical lymph nodes. Sci Rep. https://doi.org/10.1038/s41598-018-25666-4
Ergin FG (2017) Dynamic masking techniques for particle image velocimetry. Isi Bilim Tek Der 37(2):61–74
Ergin FG (2017) Dynamic masking techniques for particle image velocimetry. Isi Bilim Tek Derg 37(2):61–74
Faghih MM, Sharp MK (2021) Mechanisms of tracer transport in cerebral perivascular spaces. J Biomech 118:110278
Gülan U, Lüthi B, Holzner M, Liberzon A, Tsinober A, Kinzelbach W (2012) Experimental study of aortic flow in the ascending aorta via particle tracking velocimetry. Exp Fluids 53:1469–1485
Hablitz LM, Nedergaard M (2021) The glymphatic system. Curr Biol 31(20):1371–1375. https://doi.org/10.1016/j.cub.2021.08.026
Hablitz LM, Plá V, Giannetto M, Vinitsky HS, Stæger FF, Metcalfe T, Nguyen R, Benrais A, Nedergaard M (2020) Circadian control of brain glymphatic and lymphatic fluid flow. Nat Commun 11(1):4411
Hablitz LM, Vinitsky HS, Sun Q, Stæger FF, Sigurdsson B, Mortensen KN, Lilius TO, Nedergaard M (2019) Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Science Adv 5(2):5447
Hadaczek P et al (2006) The “perivascular pump’’ driven by arterial pulsation is a powerful mechanism for the distribution of therapeutic molecules within the brain. Mol Ther 14(1):69–78
Hand A, Sun T, Barber D, Hose D, MacNeil S (2009) Automated tracking of migrating cells in phase-contrast video microscopy sequences using image registration. J Microsc 234(1):62–79
Hill DL, Batchelor PG, Holden M, Hawkes DJ (2001) Medical image registration. Phys Med Biol 46(3):1
Hilsenbeck O, Schwarzfischer M, Skylaki S, Schauberger B, Hoppe PS, Loeffler D, Kokkaliaris KD, Hastreiter S, Skylaki E, Filipczyk A et al (2016) Software tools for single-cell tracking and quantification of cellular and molecular properties. Nat Biotechnol 34(7):703–706
Hladky SB, Barrand MA (2022) The glymphatic hypothesis: the theory and the evidence. Fluids Barriers CNS 19(1):1–33
Holstein-Rønsbo S, Gan Y, Giannetto MJ, Rasmussen MK, Sigurdsson B, Beinlich FRM, Rose L, Untiet V, Hablitz LM, Kelley DH, Nedergaard M (2023) Glymphatic influx and clearance are accelerated by neurovascular coupling. Nat Neurosci 26:1042–1053
Hussain R, Tithof J, Wang W, Cheetham-West A, Song W, Peng W, Kim D, Sun Q, Peng S, Plá V, Kelley DH, Hirase H, Castorena-Gonzalez JA, Weikop P, Goldman SA, Davis MJ, Nedergaard M (2023). Potentiating glymphatic drainage minimizes post-traumatic cerebral edema. Submitted
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid \(\upbeta\). Sci Transl Med 4(147):111–147
Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y et al (2013) Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci 33(46):18190–18199
Jessen NA, Munk ASF, Lundgaard I, Nedergaard M (2015) The glymphatic system: a beginner’s guide. Neurochem Res 40(12):2583–2599
Jonas S, Bhattacharya D, Khokha MK, Choma MA (2011) Microfluidic characterization of cilia-driven fluid flow using optical coherence tomography-based particle tracking velocimetry. Biomed Opt Express 2(7):2022–2034
Katz J, Sheng J (2010) Applications of holography in fluid mechanics and particle dynamics. Annu Rev Fluid Mech 42:531–555
Kelley DH, Ouellette NT (2011) Using particle tracking to measure flow instabilities in an undergraduate laboratory experiment. Am J Phys 79(3):267–273
Kelley DH, Thomas JH (2022) Cerebrospinal fluid flow. Annu Rev Fluid Mech. https://doi.org/10.1146/annurev-fluid-120720-011638
Kim D, Gan Y, Nedergaard M, Kelley DH, Tithof J (2023) Image Analysis Techniques for In Vivo Quantification of Cerebrospinal Fluid Flow. Zenodo. https://doi.org/10.5281/zenodo.8165799
Kiviniemi V, Wang X, Korhonen V, Keinänen T, Tuovinen T, Autio J, LeVan P, Keilholz S, Zang Y-F, Hennig J, Nedergaard M (2015) Ultra-fast magnetic resonance encephalography of physiological brain activity - glymphatic pulsation mechanisms? J Cereb Blood Flow Met 36(6):1033–1045. https://doi.org/10.1177/0271678x15622047
Li Y, Amili O, Coletti F (2022) Experimental study of concentrated particle transport in successively bifurcating vessels. Phys Rev Fluids 7(8):083101
Li G, Cao Y, Tang X, Huang J, Cai L, Zhou L (2022) The meningeal lymphatic vessels and the glymphatic system: Potential therapeutic targets in neurological disorders. J Cerebr Blood F Met 42(8):1364–1382
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS et al (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523(7560):337–341
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J (2016) Structural and functional features of central nervous system lymphatic vessels. Nature 533(7602):278–278. https://doi.org/10.1038/nature16999
Ma Q, Ineichen BV, Detmar M, Proulx ST (2017) Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat Commun 8(1):1434
Ma Q, Ries M, Decker Y, Müller A, Riner C, Bücker A et al (2019) Rapid lymphatic efflux limits cerebrospinal fluid flow to the brain. Acta Neuropathol 137(1):151–165
Manzo C, Garcia-Parajo MF (2015) A review of progress in single particle tracking: from methods to biophysical insights. Rep Prog Phys 78(12):124601
Mesquita SD, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, Contarino C, Onengut-Gumuscu S, Farber E, Raper D, Viar KE, Powell RD, Baker W, Dabhi N, Bai R, Cao R, Hu S, Rich SS, Munson JM, Lopes MB, Overall CC, Acton ST, Kipnis J (2018) Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 560(7717):185–191. https://doi.org/10.1038/s41586-018-0368-8
...Mestre H, Du T, Sweeney AM, Liu G, Samson AJ, Peng W, Mortensen KN, Stæger FF, Bork PAR, Bashford L, Toro ER, Tithof J, Kelley DH, Thomas JH, Hjorth PG, Martens EA, Mehta RI, Solis O, Blinder P, Kleinfeld D, Hirase H, Mori Y, Nedergaard M (2020) Cerebrospinal fluid influx drives acute ischemic tissue swelling. Science 367(6483):eaax7171
Mestre H, Kostrikov S, Mehta RI, Nedergaard M (2017) Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin Sci 131(17):2257–2274
Mestre H, Tithof J, Du T, Song W, Peng W, Sweeney AM, Olveda G, Thomas JH, Nedergaard M, Kelley DH (2018) Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun 9(1):4878
Milhorat TH (1975) The third circulation revisited. J Neurosurg 42(6):628–645
Min J, Rouanet J, Martini AC, Nashiro K, Yoo HJ, Porat S, Cho C, Wan J, Cole SW, Head E et al (2023) Modulating heart rate oscillation affects plasma amyloid beta and tau levels in younger and older adults. Sci Rep 13(1):3967
Moore JE Jr, Bertram CD (2018) Lymphatic system flows. Annu Rev Fluid Mech 50:459–482
Murtha LA, Yang Q, Parsons MW, Levi CR, Beard DJ, Spratt NJ, McLeod DD (2014) Cerebrospinal fluid is drained primarily via the spinal canal and olfactory route in young and aged spontaneously hypertensive rats. Fluids Barriers CNS 11(1):1–9
Møllgård K, Beinlich FR, Kusk P, Miyakoshi LM, Delle C, Plá V, Hauglund NL, Esmail T, Rasmussen MK, Gomolka RS et al (2023) A mesothelium divides the subarachnoid space into functional compartments. Science 379(6627):84–88
Møllgård K, Beinlich FR, Kusk P, Miyakoshi LM, Delle C, Plá V, Hauglund NL, Esmail T, Rasmussen MK, Gomolka RS et al (2023) A mesothelium divides the subarachnoid space into functional compartments. Science 379(6627):84–88
Nedergaard M, Goldman SA (2020) Glymphatic failure as a final common pathway to dementia. Science 370(6512):50–56
Norwood JN, Zhang Q, Card D, Craine A, Ryan TM, Drew PJ (2019) Anatomical basis and physiological role of cerebrospinal fluid transport through the murine cribriform plate. eLife 8:44278
Ouellette NT, Xu H, Bodenschatz E (2006) A quantitative study of three-dimensional lagrangian particle tracking algorithms. Exp Fluids 40:301–313
Ozbay BN, Futia GL, Ma M, Bright VM, Gopinath JT, Hughes EG, Restrepo D, Gibson EA (2018) Three dimensional two-photon brain imaging in freely moving mice using a miniature fiber coupled microscope with active axial-scanning. Sci Rep 8(1):8108
Piyawattanametha W, Cocker ED, Burns LD, Barretto RP, Jung JC, Ra H, Solgaard O, Schnitzer MJ (2009) In vivo brain imaging using a portable 2.9 g two-photon microscope based on a microelectromechanical systems scanning mirror. Opt Lett 34(15):2309–2311
Plog BA, Mestre H, Olveda GE, Sweeney AM, Kenney HM, Cove A, Dholakia KY, Tithof J, Nevins TD, Lundgaard I et al (2018) Transcranial optical imaging reveals a pathway for optimizing the delivery of immunotherapeutics to the brain. JCI Insight 3(20):e120922
Proulx ST (2021) Cerebrospinal fluid outflow: a review of the historical and contemporary evidence for arachnoid villi, perineural routes, and dural lymphatics. Cell Mol Life Sci 78(6):2429–2457
Raghunandan A, Ladron-de-Guevara A, Tithof J, Mestre H, Du T, Nedergaard M, Thomas JH, Kelley DH (2021) Bulk flow of cerebrospinal fluid observed in periarterial spaces is not an artifact of injection. eLife 10:65958
Ramos M, Burdon Bechet N, Battistella R, Pavan C, Xavier ALR, Nedergaard M, Lundgaard I (2019) Cisterna magna injection in rats to study glymphatic function 97–104
Rasmussen MK, Mestre H, Nedergaard M (2018) The glymphatic pathway in neurological disorders. Lancet Neurol 17(11):1016–1024
Rasmussen MK, Mestre H, Nedergaard M (2021) Fluid transport in the brain. Physiol Rev 102(2):1025–1151
Ray LA, Heys JJ (2019) Fluid flow and mass transport in brain tissue. Fluids 4(4):196
Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA (1985) Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res 326(1):47–63
Rey J, Sarntinoranont M (2018) Pulsatile flow drivers in brain parenchyma and perivascular spaces: a resistance network model study. Fluids Barriers CNS 15(1):20
Ringstad G, Valnes LM, Dale AM, Pripp AH, Vatnehol S-AS, Emblem KE, Mardal K-A, Eide PK (2018) Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI Insight 3(13):e121537
Salminen AT, Tithof J, Izhiman Y, Masters EA, McCloskey MC, Gaborski TR, Kelley DH, Pietropaoli AP, Waugh RE, McGrath JL (2020) Endothelial cell apicobasal polarity coordinates distinct responses to luminally versus abluminally delivered TNF-\(\alpha\) in a microvascular mimetic. Integr Biol 12(11):275–289
Schley D, Carare-Nnadi R, Please CP, Perry VH, Weller RO (2006) Mechanisms to explain the reverse perivascular transport of solutes out of the brain. J Theor Biol 238(4):962–974
Sengupta PP, Pedrizetti G, Narula J (2012) Multiplanar visualization of blood flow using echocardiographic particle imaging velocimetry. JACC Cardiovasc Imag 5(5):566–569
Shanbhag NC, Bèchet NB, Kritsilis M, Lundgaard I (2021) Impaired cerebrospinal fluid transport due to idiopathic subdural hematoma in pig: an unusual case. BMC Vet Res 17:1–8
Smith AJ, Verkman AS (2018) The “glymphatic’’ mechanism for solute clearance in Alzheimer’s disease: game changer or unproven speculation? FASEB J 32(2):543–551
So PT, Dong CY, Masters BR, Berland KM (2000) Two-photon excitation fluorescence microscopy. Annu Rev Biomed Eng 2(1):399–429
Spector R, Snodgrass SR, Johanson CE (2015) A balanced view of the cerebrospinal fluid composition and functions: Focus on adult humans. Exp Neurol 273:57–68
Spera I, Cousin N, Ries M, Kedracka A, Castillo A, Aleandri S, Vladymyrov M, Mapunda JA, Engelhardt B, Luciani P, Detmar M, Proulx ST (2023) Open pathways for cerebrospinal fluid outflow at the cribriform plate along the olfactory nerves. EBioMedicine 91
Steffensen AB, Edelbo BL, Barbuskaite D, Andreassen SN, Olsen MH, Møller K, MacAulay N (2023) Nocturnal increase in cerebrospinal fluid secretion as a circadian regulator of intracranial pressure. Fluids Barriers CNS 20(1):1–14
Sweeney AM, Plá V, Du T, Liu G, Sun Q, Peng S, Plog BA, Kress BT, Wang X, Mestre H et al (2019) In vivo imaging of cerebrospinal fluid transport through the intact mouse skull using fluorescence macroscopy. JoVE-J Vis Exp 149:e59774
Tithof J, Kelley DH, Mestre H, Nedergaard M, Thomas JH (2019) Hydraulic resistance of periarterial spaces in the brain. Fluids Barriers CNS 16(19):1–13
Tithof J, Boster KAS, Bork PAR, Nedergaard M, Thomas JH, Kelley DH (2022) A network model of glymphatic flow under different experimentally-motivated parametric scenarios. iScience
Vennemann P, Lindken R, Westerweel J (2007) In vivo whole-field blood velocity measurement techniques. Exp Fluids 42:495–511
Vindedal GF, Thoren AE, Jensen V, Klungland A, Zhang Y, Holtzman MJ, Ottersen OP, Nagelhus EA (2016) Removal of aquaporin-4 from glial and ependymal membranes causes brain water accumulation. Mol Cell Neurosci 77:47–52
Vinje V, Eklund A, Mardal K-A, Rognes ME, Støverud K-H (2020) Intracranial pressure elevation alters csf clearance pathways. Fluids Barriers CNS 17(1):1–19
Wang H, Li Z, Zhang X, Zhu L, Liu Y, Wang S (2020) The motion of respiratory droplets produced by coughing. Phys Fluids 32(12):125102
Wang P, Olbricht WL (2011) Fluid mechanics in the perivascular space. J Theor Biol 274(1):52–57
Weller RO (1998) Pathology of cerebrospinal fluid and interstitial fluid of the CNS: significance for Alzheimer disease, prion disorders and multiple sclerosis. J Neuropath Exp Neur 57(10):885–894
Wright BL, Lai JT, Sinclair AJ (2012) Cerebrospinal fluid and lumbar puncture: a practical review. J Neurol 259(8):1530–1545
Xavier AL, Hauglund NL, Holstein-Rathlou S, Li Q, Sanggaard S, Lou N, Lundgaard I, Nedergaard M (2018) Cannula implantation into the cisterna magna of rodents. JoVE-J Vis Exp 135:57378
**e L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M (2013) Sleep drives metabolite clearance from the adult brain. Science 342(6156):373–377
Yamada M (2015) Cerebral amyloid angiopathy: emerging concepts. J Stroke 17(1):17
Yardeni T, Eckhaus M, Morris HD, Huizing M, Hoogstraten-Miller S (2011) Retro-orbital injections in mice. Lab Animal 40(5):155–160
You J, Mallery K, Hong J, Hondzo M (2018) Temperature effects on growth and buoyancy of microcystis aeruginosa. J Plankton Res 40(1):16–28
You J, Mallery K, Mashek DG, Sanders M, Hong J, Hondzo M (2020) Microalgal swimming signatures and neutral lipids production across growth phases. Biotechnol Bioeng 117(4):970–980
Acknowledgements
We thank Kim Boster for valuable insight and suggestions, as well as substantial contributions to the development of our MATLAB visualization tool “imagei.m.” We also thank Keelin Quirk for implementing more advanced kinematic predictions in our particle tracking MATLAB script “PredictiveTracker.m.”
Funding
DK and JT are supported by a Career Award at the Scientific Interface from Burroughs Wellcome Fund. YG, MN, and DHK are supported by NIH BRAIN Initiative U19NS128613, NIH National Center for Complementary and Integrative Health R01AT012312, and US Army MURI W911NF1910280.
Author information
Authors and Affiliations
Contributions
JT, DHK, and MN helped in conceptualization; JT and DHK helped in methodology; DK and YG helped in formal analysis and investigation; DK, JT, and DHK contributed to writing—original draft preparation; JT and DHK contributed to writing—review and editing; JT, DHK, and MN worked in funding acquisition; JT, DHK, and MN worked in resources; and JT, DHK, and MN worked in supervision.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical Approval
Animal experiments were approved by the Danish Animal Experiments Inspectorate or the University Committee on Animal Resources of the University of Rochester and performed according to guidelines from the National Institutes of Health (NIH).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix A Particle tracking uncertainty analysis
Appendix A Particle tracking uncertainty analysis
In this Appendix, we analytically calculate the approximate uncertainty in particle tracking velocity measurements using propagation of error based on estimated uncertainties in particle position and image acquisition time. The numbers used here are specific for one particular data set (Kim et al. 2023), serving as a concrete example, but the reader could readily repeat these calculations for different imaging parameters. This derivation applies to two-photon microscopy, wherein each 2D image is assembled over a finite window in time during which each pixel is individually rastered. Hence, for images acquired at 29.6 Hz, each acquired image has an uncertainty of approximately \(\Delta t=33.8\) ms. Note this value is an upper bound for the temporal uncertainty, this upper bound will generally be larger/smaller when a greater/lesser number of pixels are recorded, and more precise (smaller) values of \(\Delta t\) could be obtained by accounting for details of the TPM rastering technique.
The velocity of a tracked particle in frame n can be estimated, to the first order, as \(U_n = \frac{\Delta x}{\Delta t}\), where \(\Delta x\) is the measured displacement of the particle since frame \(n-1\), and \(\Delta t\) is the measured time elapsed since frame \(n-1\). Denoting the error in \(\Delta x\) as \(u_x\) and the error in \(\Delta t\) as \(u_t\), the error in \(U_n\) is
Our image processing algorithm locates each particle by finding the centroid of a contiguous bright region above a given threshold, typically achieving error on the order of 0.1 pixel. In these data, each pixel has lateral dimension \(1.04~\upmu \hbox {m}\), so we estimate \(u_x \approx 0.104~\upmu \hbox {m}\). As mentioned above, for a frame rate of 29.6 Hz, \(\Delta t = 33.8\) ms. Now suppose the root-mean-square single-frame displacement is \(\Delta x = 2.158~\upmu \hbox {m}\) (this value is obtained empirically by performing particle tracking). Estimating the timing error \(u_t\) for a two-photon microscope is subtle because images are produced not by making simultaneous measurements from an array of sensors but by making subsequent measurements as the single focal point rasters the field of view. If the focal point traces column-by-column, then measuring a particle moving to an adjacent column involves greater timing error than measuring a particle moving to an adjacent row. Considering image dimensions \(512 \times 512\) pixels, the two timing errors would be roughly \(\Delta t / 512\) and \(\Delta t / 512^2\), respectively. To be conservative, we take \(u_t \approx \Delta t / 512 = 66.0~\upmu \hbox {s}\). Using these values, we find \(\epsilon _U = 3.08~\upmu \hbox {m/s}\).
In practice, we estimate the velocity with a higher-order method, convolving the measured position with a kernel that provides differentiation and smoothing. Explicitly, the numerical scheme used in our code makes the estimate
where \(\alpha = 2 / (\pi ^{1/2} \textrm{erf}(3) - 6 e^{-9}) \approx 1.129\) and
The velocity error is
where \(u_{n-3}\) is the measurement error associated with location \(x_{n-3}\), \(u_{n-2}\) is the measurement error associated with location \(x_{n-2}\), and so on. Assuming homogeneity implies that all those errors have the same value, which we again denote \(u_x\). Then, the velocity error becomes
where \(\beta = 18e^{-18} + 8e^{-8} + 2e^{-2} \approx 0.273.\) To estimate the value of \(\Delta {\widetilde{x}}\), we consider the case in which a particle’s displacement between any two frames is the measured root-mean-square value \(\Delta x\), implying \(x_{n+1} - x_{n-1} = 2 \Delta x\), \(x_{n+2} - x_{n-2} = 4 \Delta x\), and \(x_{n+3} - x_{n-3} = 6 \Delta x\). Therefore, \(\Delta {\widetilde{x}} = \gamma ^{1/2} \Delta x,\) where \(\gamma = (2 e^{-1} + 8 e^{-4} + 18 e^{-9})^2 \approx 0.782\). Altogether, the velocity error is
Comparing to Eq. (A1), we see that the error in velocity estimated with the higher-order numerical scheme differs from the error in the first-order velocity estimates only by factors of order unity. Again taking the same values for \(u_t\), \(u_x\), \(\Delta t\), and \(\Delta x\), the velocity error in the higher-order scheme is \(\epsilon _U = 1.82~\upmu \hbox {m/s}\), about 40% lower than with the first-order estimate.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, D., Gan, Y., Nedergaard, M. et al. Image analysis techniques for in vivo quantification of cerebrospinal fluid flow. Exp Fluids 64, 181 (2023). https://doi.org/10.1007/s00348-023-03719-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00348-023-03719-3