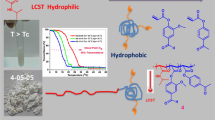
Abstract
This work has been done in three steps. First, the preparation of acrylate monomers; they are [dimethyl-1,3-dioxoylan-4-yl-methylacrylate (sol-ketal acrylate) (SKA)], [4-acetylphenyl acrylate (APHA)], and [4-formyl-2-methoxyphenylacrylate (VA)]. All monomers were evaluated using 1H, 13C-NMR, and FT-IR. In the next step, two kinds of polymers were prepared. Two series of copolymers and terpolymers were carried out via the free-radical polymerization; SKA with the photo-cross-linker for poly (SKA-co-DMIAm) photo-cross-linker polymer and VA and APHA with N-isopropylacrylamide for poly (NIPA-co-VA-co-APHA) functional-thermo-responsive terpolymer. All fabricated polymers were investigated by (1H-NMR, FT-IR, UV, GPC, and DSC). The phase separation temperature of polymer solutions has been measured through the turbidity and the change in transmittance to the temperatures using UV–Vis spectroscopy. Eventually, the UV was used to form the gel layer after the deposition of the gold layer. The nonresponsive gel layer was grafted with poly (NIPA-co-VA-co-APHA) to optimize the upper layer to the thermo-responsive functional layer. SPR/OW measured the swelling properties of the gel layers. The active layer will immobilize biological molecules with the primary amine group.
Graphical abstract
The schematic diagram shows the steps of gel formation: The cross-linking initiated by UV; SPR/OW for film thickness; grafting for gel optimization.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently materials with unique properties that probably alter their properties with the response to their outer environment have been of great interest. These polymers and hydrogel materials were called environmental, responsive, stimuli-responsive, and smart [1,2,3,4,59]. It has been added to the supplanted material.
Preparation of (4-acetylphenyl acrylate) (APHA) [60]
It has been synthesized as described in reference [58]. It has been added to the supplanted material.
Preparation of vanillin acrylate or [4-formyl-2-methoxyphenylacrylate] (VA).
It has also been prepared, as reported in recent articles [60]. It has been added to the supplemented material.
Preparation of photo-cross-linker and adhesion
Dimethylmaleimidoacrylate (DMIA) photo-cross-linker
The method was used as described in the recent lecture [2, 61], discussed in detail in the supplemented material.
Preparation of [3-(3,4-dimethyl-2,5-dioxo-2,5-dihydro-pyrrol-yl)-propyl ester] adhesion (DMITAc),
It is discussed in detail in the supplemented material. The method was used as described in the recent lecture [2].
Fabrication of copolymers and terpolymers
Poly (SKA-co-DMIA)
Three round flasks 5 mol% (0.125 g, 0.383 mmol), 10 mol%, 0.325 g, (0.767 mmol), and 15 (0.375 g, 1.149 mmol) of (DMIA), respectively, 2.00 g (0.01 mol) SKA, and AIBN as initiator was dissolved in 50 mL 1,4-dioxane. They purged in argon for 15 min. and heated at 65 ℃ for 8 h. The polymer was extracted in diethyl ether, at − 50 ℃, a viscous oil material purified in THF.
1H-NMR (Chloroform-d), δ(ppm) = [1.28–1.44 (m, 6-H, j-2-CH3), 1.45–1.77(m, 3-H, f-CH, e-CH2), 1.91–2.03 (m, 6-H, a-2-CH3), 2.17–2.47 (m, 3-H, d-CH2, c–CH), 3.61–3.84 (m, 2-H, g-CH2), 3.92–4.18 (m, 2-H, i-CH2), 4.20–4.37 (m, 1-H, h-CH)].
FT-IR (KBr), ν (cm−1) = [2930–2976 (s) (CH, CH2 and CH3-Aliphatic), 1735–1747(s) (C = O, ester), 1655–1660 (s) (C = C maleimide).
Poly (NIPA-co-VA-co-APHA) terpolymer
100-mL round flasks, mixtures of 0.167, 0.334, 0.501 g corresponding to (5, 10, 15 mol%) of (APHA) were added to 10 mol% 0.362 g (VA), respectively, and (0.0176 mol) 2.00 g NIPA in 50 mL of 1,4-dioxane and 10–3 mol AIBN, and then purged in argon for 15 min. The reaction vessel was heated 8 h at 70 ℃. After cooling at room temperature and in a refrigerator, the polymer was precipitated in diethyl ether at − 60 ℃. The terpolymers were purified in THF. The product was dried under reduced pressure as a yellowish solid.
1H-NMR (Chloroform-d), δ(ppm) = 0.78–1.34 (m, 6-H, m-2-CH3), 1.42–2.45 (m, 9-H, c-h -CH2, -CH), 2.46–2.65 (m, 3-H, a-CH3), 3.64–3.72 (m, 3-H, j-CH3), 3.79–4.18 (m, 1-H, n–CH), 5.87–6.73 (m, 1-H, l-NH), 7.10–8.13 (m, 8-H, b, i-CH-Ar.), 9.85–10.07 (m, 1-H, k-HCO).
FT-IR (KBr), ν (cm−1) = 3365 (NH group), 2979–3070 (Aliphatic CH, CH2, and CH3), 1763 (s) (C = O in –COO ester group), 1692 (s) (HC = O, carbonyl aldehyde), 1571 (s) (C = O in CONH amide), 1105 (m) (CH3 in OCH3), 862 (m) (CH-Aromatic).
Preparation of hydrogel thin film
Fabrication of gel thin films based on poly (SKA-co-DMIA)
A slide of LaSFN9 glass (2 cm. H × 2 cm. W) was cleaned with pure ethanol and dried under reduced pressure. 45–50 nm of pure gold has been evaporated and then immersed in a solution of 5 mM of adhesion [3-(3,4-dimethyl-2,5-dioxo-2,5-dihydro-pyrrol-yl)-propyl ester (DMITAc)] 0.10 wt% poly (SKA-co-DMIA) polymer solution with (0.0005 wt%) thioxanthone in distilled cyclohexanone was spin-coated over LaSFN9 + DMITAc (2000 rpm). The coated slide has been exposed to UV irradiation through a UV lamp (300–430 nm). The cyclodimerization [2 + 2] between dimethyl maleimide groups and the photo-cross-linking is formed in Fig. 5 (8a).
Ring-opening of [2,2-dimethyl-1,3-dioxoylan-4-yl-methylacrylate (SKA) to 2,3-dihydroxypropyl acrylate] (DHPA)
100-mL flask, fixed with a reflux condenser, the spin-coated glass slide was prepared in step A and immersed in a mixture of [20 mL glacial acetic acid and 15 mL THF] and refluxed for 6 h at 90 ℃. Afterward, the slide was picked up and cleaned from THF, DI H2O, and dried using vacuum nitrogen. The ATR has measured the change of functional groups by the chemical modification on the surface; the (SPR/OW) has been used to determine the refractive index. The film thickness of the gel in the dry and swelling states was calculated and drawn in Fig. 6 (8b).
ATR-IR ν (cm−1) = 3870–4105 (s, br. multiple peaks) (OH), 3550–3760 (s, br. multiple peaks) (CH, CH2, and CH3 multiple peaks aliphatic), 1675–1745 (br. multiple peaks) (–C = O), 1560–1578 (s, multiple peaks) (–C = C–).
Surface grafting poly (DHPA-co-DMIA)-g-(NIPA-co-APHA-co-VA).
The coated slide by poly (DHPA-co-DMIA) gel has been immersed in a poly (NIPA-co-APHA-co-VA) in 30 mL CHCl3 dry. 0.2 g (1.16 mmol) of p-toluenesulfonic acid has also been added to 100-mL flask. They were refluxed at 80℃ for 4 h. They were cold, and 0.2 g of Na2CO3 was added with reflux for about 30 min. The gel was cleaned and dried, as discussed previously. It was chemically investigated by ATR and the film thickness by (SPR-OW).
ATR-IR ν (cm−1) = 3530–3720 (m, br. multiple peaks) (CH, CH2, and CH3 aliphatic), 2270–2575 (s) (O-C-O), 1675–1745 (br. multiple peaks) (-C = O), 1565–1595 (s, multiple peaks) (-C = C-), 720–830 (m) (CH-Ar.).
Swelling measurements
The film thickness for each layer and swelling properties has been scanned by surface plasmon resonance with an optical waveguide. The change in refractive indexes RI (θ) was used to intemperate the degree of swelling. Knoll et al. discussed the relationship between the waveguide and film thickness and demonstrated an indirect relation as the higher waveguide modes with lower incident angles and vice versa [53]. However, the plasmon minimum can be displaced from ~ 65.5° to ~ 68°, indicating the phase separation from the swollen to collapse. By simulations and Fresnel calculations, the relation of volume degree of swelling or refractive index with temperature was used to detect the phase separation temperature of the hydrogel.
Results and discussion
Fabrication of monomers
This work uses the preparation of three hydrophobic monomers with and without functionality as described in Scheme 1. [2,2-dimethyl-1,3-dioxolane-4-yl-methylacrylate] (sol-ketal acrylate) (SKA) has been synthesized in two steps, the first is the formation of isopropylideneglycerol (1) by the reaction of acetone and glycerol in acidic media the product was chemically evaluated. The 1H-NMR showed 2CH3 groups at δ = 1.27 and 1.34 ppm, and a broad peak at δ = 2.75 ppm for the OH group. The 13C-NMR and FT-IR have also been used in supplemented material. The final product [2,2-dimethyl-1,3-dioxoylan-4-yl-methylacrylate] compound (2) has been prepared by reacting (1) with acryloyl chloride and triethylamine. It was investigated by both 1H-NMR and 13C, representing the presence of 3-H of the vinyl group (–CH = CH2) at δ = 5.8, 6.0, and 6.3 ppm corresponding to δ = 109, 127, and 131 ppm. The FT-IR showed the essential peak for the C = C vinyl group at υ = 1660 cm−1. All data have been added to the supplemented material. For monomers (3) (APHA) and (4) (VA), they were prepared as mentioned in our recent articles [58]. They were investigated by 1H-NMR, 13C, and FT-IR and demonstrated good results with their chemical structures. These results have also been added to the supplemented material.
Fabrication of polymers gel thin films
Here, we are concerned with fabricating a series of functional photo-cross-linked copolymers from SKA with different concentrations of DMIAm (6) and another functional thermos-responsive terpolymers series based on N-isopropylacrylamide. They have been implemented by free-radical polymerization in a solution using (AIBN) for the initiation process as Scheme 2. The chemical investigation occurred by the 1H-NMR and FT-IR, which agreed well with their chemical structures. The monomer concentrations after polymerization were calculated from the 1H-NMR integration of 6-H, 2-CH3 of SKA at δ = 1.28 to 1.44 ppm, at δ = 1.91–2.03 6-H of the cross-linker DMIAm, δ = 3.61–3.84 ppm 2-H of SKA and at δ = 3.92–4.18 ppm 2-H of SKA ring, as shown in Table 1 and Figs. 1, 3. NIPA, VA, and APHA terpolymers with different molar ratios of 5, 10, and 15 of APHA and 10 molar concentrations of VA (7) have also been prepared as illustrated in Scheme 2. The presence of ketone and aldehyde groups for each APHA and VA, respectively, encourages the formation of other chemical reactions; it will be discussed in the next step. 1H-NMR and FT-IR have investigated the chemical structures, exhibiting the absence of vinyl groups of monomers and an active aldehyde group, as shown in Figs. 2 and 3. The concentrations of each monomer were determined by the 1H-NMR integration of specific protons at δ = 4.18 ppm 1-H, CH for NIPA, and at δ = 2.56 ppm 3-H, CH3 APHA, and at δ = 10.07 ppm 1-H, CHO, VA as shown in Table 1. The FT-IR of solid polymers with dry KBr illustrated some specific peaks related to functional groups for polymer (6) at υ = 1747 cm−1 –C = O ester, 1660 cm−1 –C = C– cross-linker), while polymer (7) at υ = 1763 cm−1 –C = O, carbonyl ester, 1692 cm−1 –C = O, aldehyde, 1571 cm−1 –C = O amide as shown in Fig. 3.
The process has been investigated by applying the surface to ZnSe ATR crystal (Fig. 4). The surface of the gel was exposed to the chemical reaction and ring-opening of SKA, and then ring closure with APHA and cross-linking. Figure 5 shows the formation of -OH groups at 3870–4105 (s) multiple peaks; otherwise, the disappearance of these peaks after cross-linking with APHA is shown in Fig. 6.
Polymer characterizations
Size exclusion chromatography technique (SEC) or gel permeation chromatography (GPC) has been implemented to display the molecular weights (Mn) and dispersity (Ð) as well, in dimethylacetamide (DMA) as an eluent and polystyrene (PS) column; the concentration used 6 g/L. Figure 7 shows the relation between the weight average molecular weight with log [M]. It demonstrates one peak for each polymer, emphasizing polymer formation and the disappearance of impurities and small molecules like monomers [11]. Another observation pointed in the dispersity demonstrated a decrease in Ð by increasing the photo-cross-linker in the polymer (6) and VA and APHA for polymer (7) interpreted the free-radical polymerization and formation of copolymers and terpolymers for 6 and 7, respectively [11]. The glassy temperatures (Tg,s) were recorded as the second-order change in the heat flow using differential scanning calorimetry (DSC). The DSC thermograms represent decreasing in Tg − 23, − 28, and − 37 ℃ of polymers 6a-05, 6a-10, and 6a-15, respectively, due to the high hydrophobicity of both SKA and DMIAm in the polymer main chain, which decreases stiffness and increases the flexibility [59] Fig. 8. Alternatively, polymers 7a-05, 7a-10, and 7a-15 copolymerized with N-isopropylacrylamide have higher glass transition temperature Tg attributed to the amide group's presence, increasing the hydrophilicity in the polymer chain, further increasing stiffness, and decrease flexibility Fig. 9. The phase separation demonstrated in the lower critical solution temperature of temperature-responsive polymers 7a-05, 7a-10, and 7a-15 showed a lower value of Tc than N-isopropylacrylamide homopolymer. Figure 10 shows the relation between temperature and transmittance of polymers 7a-05, 7a-10, and 7a-15, and the Tc was determined at the inflection point exhibited 18 ℃, 15 ℃, and 13 ℃, respectively; the lowest Tc was detected with the higher concentration of APHA and VA that attributed to its higher hydrophobicity which faster the phase separation in solution [62].
Gel thin films
This study aims to modify the surface of the hydrophobic layer based on PSKA photo-cross-linked gel film. The modification process involved three steps; SPR/OWS described each step for dry and swollen states.
Formation of the hydrophobic gel layer
PSKA gel thin film was fabricated using a solution of poly (SKA-co-DMIA) (10 mol% of DMIA) dissolved in distilled cyclohexanone at (2000 rpm). The dry film was scanned by (SPR/OW). It demonstrated the absence of attenuated total reflection (ATR) and the presence of waveguide at 25°, indicating the homogenous film's formation. Fresnel calculations of the dry thickness showed 264 nm and 1.473 refractive index unit (RIU) in Table 2 and Fig. 11. The hydrophobic environment has been observed from the non-swelling of gel films measured via SPR/OW and using deionized water. The relation of the refractive indexes (RI) with temperature variations has occurred. Fresnel calculations were used to detect the RI for the volume degree of swelling (1/χp). Figure 12 shows the relationship between (1/χp) and RI with temperature, and the surface demonstrated unaffected by temperature.
Conversion to the hydrophilic gel layer
The hydrophobic gel layer has been optimized to a hydrophilic one by releasing the hydroxyl groups of SKA, as mentioned later. The chemical structure was investigated by ATR administrated logic results. The overall process has been schematically drawn in Scheme 3. SPR/OWS has investigated the surface for dry and swell film thickness. Fresnel calculations for the dry layer exhibited 250 nm (film thickness) and ~ 1.476 RIU with a waveguide at θ = 23, as shown in Fig. 11. The swelling was measured in deionized water. The scanning process has been performed with the change of temperature. An increase in (1/χp) from ~ 1.6 to ~ 1.7 corresponds to a decrease in RI from ~ 1.39 RIU to ~ 1.38 RIU by raising the temperature due to anisotropic behavior. Figure 13 illustrates the change in (1/χp) and RI with temperature as an independent and demonstrates the anisotropic behavior.
Formation of the temperature-responsive gel layer
The gel layer was converted to a hydrophilic in the previous step, and free hydroxyl groups were formed. Poly (NIPA-co-VA-co-APHA) (7b-10–10) was dissolved in cyclohexanone and then coated to the gel layer that was further chemically cross-linked, as discussed in the experimental section and described in Scheme 3.
The SPR/OWS scan for the dry state showed waveguide mode with ATR. Fresnel calculations for a single dry layer showed 320 nm (film thickness) and RI ~ 1.466 RIU, as shown in Fig. 11, indicating the additional thin film. After swelling in deionized water, the scanning of SPR with OW has been achieved, demonstrating the change of RI (θ) with temperature, as illustrated in Fig. 14. Some essential features have been recorded;
-
A-
The minimum line of plasmon was shifted from ~ 75 to ~ 77 by raising the temperature.
-
B-
Higher (1/χp) than detected in the previous step.
-
C-
The refractive index was lower than in the previous step.
The phase separation temperature of the upper layer has been detected from the relation of (1/χp) or RI with temperature. It is difficult to see the change due to the hydrophobic layer covering and protecting the upper layer's sensitivity [49]. However, the higher the protection of the hydrophobic layer, the NIPA still has affection and demonstrated LCST (Tc) at approximately 20 ℃ at the inflection point, as shown in Figs. 14, 15.
Conclusion
In this study, new acrylate monomers were fabricated to act as functional and hydrophobic monomers. A series of copolymers and terpolymers were fabricated via free-radical polymerization. The copolymerization of the hydrophobic monomer, at high concentrations, with N-isopropylacrylamide resulted in lower phase transition temperatures. The photo-cross-linked sol-ketal acrylate (SKA) gel was formed over the gold substrate. Surface plasmon resonance/optical waveguide (SPR/OW) was used to measure the film thickness of the dry and swollen gels. The volume degree of swelling and refractive index showed no response to temperature variations. Furthermore, the surface of SKA was modified by ring-opening, forming hydroxyl groups to increase the hydrophilicity of the polymer gel. The free hydroxyl groups were grafted by poly (NIPA-co-VA-co-APHA). The modified gel surface showed thermo-responsive behavior, where the phase separation temperature was detected by SPR/OW. This study offers insights into the fabrication of thermo-responsive gels which can be used in bio-separation processes through the formation of gel vessels for attaching and releasing biomolecules.
References
Abdelaty MSA (2022) The influence of pH/salt concentrations on tuning lower critical solution temperature of poly(NIPAAm-co-DMAA-co-DTBAVA) multi-environmentally terpolymer. J Polym Environ 30:4130–4145t. https://doi.org/10.1007/s10924-022-02502-5
Abdelaty MSA (2019) Layer by layer photo-cross-linked environmental functional hydrogel thin films based on vanillin: part 3. J Polym Environ 21:1–16. https://doi.org/10.1007/s10924-019-01421-2
Abdelaty MSA (2019) Influence of vanillin acrylate and 4-acetylphenyl acrylate hydrophobic functional monomers on phase separation of N-isopropylacrylamide environmental terpolymer: fabrication and characterization. Polym Bull. https://doi.org/10.1007/s00289-019-02890-0
Vanessa FC, Daniela MC, Clarisse R, Margarida MF, Senentxu LM (2018) Fluorinated polymers as smart materials for advanced biomedical applications. Polymers 10:161. https://doi.org/10.3390/polym10020161
Quan T, Dinglei Z, Haiyang Y, Lijun W, **ngyuan Z (2019) A pH-responsive self-healing hydrogel based on multivalent coordination of Ni2+ with polyhistidine-terminated PEG and IDA-modified oligochitosan. J Mater Chem B 7:30–42. https://doi.org/10.1039/C8TB02360C
Guangyan Z, Xulin J (2019) Temperature responsive nanoparticles based on PEGylated polyaspartamide derivatives for drug delivery. Polymers 11:316. https://doi.org/10.3390/polym11020316
Tao X, Ting L, Wei-Feng Z, Cheng-Sheng Z (2019) Ionic-strength responsive zwitterionic copolymer hydrogels with tunable swelling and adsorption behaviors. Langmuir 35:1146–1155. https://doi.org/10.1021/acs.langmuir.8b01719
Yifan G, Ziying L, Yiru H, Lijie S, Shuo C, Zenghe L, Chuanglong H, ** B, **anqun F, Zhengwei Y (2019) A biodegradable functional water-responsive shape memory polymer for biomedical applications. J Mater Chem B 7:123–132. https://doi.org/10.1039/C8TB02462F
Moldenhauer D, Fuenzalida W, Strassert CA, Gröhn F (2019) Light-responsive size of self-assembled Spiropyran–Lysozyme nanoparticles with enzymatic function. Biomacromol. https://doi.org/10.1021/acs.biomac.8b01605
Iatridi Z, Mattheolabakis G, Avgoustakis K, TsitsilianisC, (2011) Self-assembly and drug delivery studies of pH/thermo-sensitive polyampholytic (A-co-B)-b-C-b-(A-co-B) segmented terpolymers. Soft Matter 7:11160–11168. https://doi.org/10.1039/C1SM06185B
Abdelaty MSA (2018) Preparation and characterization of new environmental functional polymers based on vanillin and Nisopropylacrylamide for post polymerization. J Polym Environ 26:636–646. https://doi.org/10.1007/s10924-017-0960-2
Abdelaty MSA (2018) Poly(N-isopropylacrylamide-co-2-((diethylamino)methyl)-4-formyl-6-methoxyphenylacrylate) environmental functional copolymers: synthesis, characterizations, and grafting with amino acids. Biomolecules 8:154. https://doi.org/10.3390/biom8040138
Abdelaty MSA (2022) Schiff base post-polymerization based on temperature/ph environmentally responsive poly (NIPAAm-co-DMAMVA-co-S): characterization and the trigger of LCST behavioral changes. Polym Bull. https://doi.org/10.1007/s00289-022-04327-7
Chang K, Rubright N, Lowery PD, Taite LJ (2013) Structural optimization of highly branched thermally responsive polymers as a means of controlling transition temperature. J Polym Sci APolym Chem 51:2068–2078. https://doi.org/10.1002/pola.26596
Heskins M, Guillet JE (1968) Solution properties of poly(N-isopropylacrylamide). J Macromol Sci Chem, A. 2:1441–1455. https://doi.org/10.1080/10601326808051910
Abdelaty MSA (2016) Kuckling D (2016) Synthesis and characterization of new functional photo-cross-linkable smart polymers containing vanillin derivatives. Gels 2:1–13
Thomas EL (2018) Nanoscale 3D ordered polymer networks. Sci China Chem 61:25–32. https://doi.org/10.1007/s11426-017-9138-5
Epps TH, Cochran EW, Bailey TS, Waletzko RS, Hardy CM, Bates FS (2004) Ordered network phases in linear poly(isoprene-b-styrene-b-ethylene oxide) triblock copolymers. Macromolecules 37:8325–8341
Abdelaty MSA, Kuckling D (2021) Altering of lower critical solution temperature of environmentally responsive poly (N-isopropylacrylamide-co-acrylic acid-co-vanillin acrylate) affected by acrylic acid, vanillin acrylate, and post-polymerization modification. Colloid Polym Sci 299:1617–1629. https://doi.org/10.1007/s00396-021-04882-x
Kaemmerer E, Melchels FP, Holzapfel BM, Meckel T, Hutmacher DW, Gelatine LD (2014) Methacrylamide-based hydrogels: an alternative three-dimensional cancer cell culture system. Acta Biomater 10:2551–2562. https://doi.org/10.1016/j.actbio.2014.02.035
Thakur VK, Kessler MR (2015) Self-healing polymer nan composite materials: a review. Polymer 69:369–383. https://doi.org/10.1016/j.polymer.2015.04.086
Deng G, Li F, Yu H, Liu F, Liu C, Sun W, Jiang H, Chen Y (2012) Dynamic hydrogels with an environmental adaptive self-healing ability and dual responsive sol–gel transitions. ACS Macro Lett 1:275–279. https://doi.org/10.1021/mz200195n
Hoare TR, Kohane DS (2008) Hydrogels in drug delivery: progress and challenges. Polymer 49:1993–2007. https://doi.org/10.1016/j.polymer.2008.01.027
Gupta P, Vermani K, Garg S (2002) Hydrogels: from controlled release to pH-responsive drug delivery. Drug Discov Today 7:569–579. https://doi.org/10.1016/S1359-6446(02)02255-9
Wei W, **nyu Hu, Qi X, HaoYu YL, Li J, Zhang J, Don W (2015) A novel thermo-responsive hydrogel based on salecan and poly(N-isopropylacrylamide): synthesis and characterization. Colloids Surf, B 125:1–11
Tang S, Floy M, Bhandari R, Sunkara M, Morris AJ, Dziubla TD, Hilt JZ (2017) Synthesis and characterization of thermoresponsive hydrogels based on N-isopropylacrylamide crosslinked with 4,4’-dihydroxybiphenyl diacrylate. ACS Omega 31:8723–8729. https://doi.org/10.1021/acsomega.7b01247
Abdelaty MSA (2022) Fluctuation in the phase transition temperature of poly (NIPAAm-co-HEMA-co-DMAMVA)-post-guanine affected by hydrophilic/hydrophobic interaction: fabrication and characterizations. J Polym Environ 30:4406–4417. https://doi.org/10.1007/s10924-022-02512-3
Saunders BR (2004) On the structure of poly(N-isopropylacrylamide) microgel particles. Langmuir 20:3925–3932. https://doi.org/10.1021/la036390v
Zheng YG, Chen XL, Shen YC (2008) Commodity chemicals derived from glycerol, an important biorefinery feedstock. Chem Rev 108:5253–5277. https://doi.org/10.1021/cr068216s
Zhou CHC, Beltramini JN, Fan YX, Lu GQM (2008) Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem Soc Rev 37:527–549. https://doi.org/10.1039/B707343G
Oguzhan I, Senol Y, and Funda O A (2017) Synthesis of sol-ketal from glycerol and acetone over amberlyst-46 to produce an oxygenated fuel additive. Periodica Polytech, Chem Eng 61:144–148. https://doi.org/10.3311/PPch.8895
Narinthorn S, Boonyarach K (2011) Synthesis of sol-ketal from glycerol and its reaction with benzyl alcohol. Energy Prcedia 9:63–69. https://doi.org/10.1016/j.egypro.2011.09.008
Save M, Weaver JVM, Armes SP (2002) Atom transfer radical polymerization of hydroxy-functional methacrylates at ambient temperature: comparison of glycerol monomethacrylate with 2-hydroxypropyl methacrylate. Macromolecules 35:1152–1159. https://doi.org/10.1021/ma011541r
Haigh R, Rimmer S, Fullwood NJ (2000) Synthesis and properties of amphiphilic networks. 1: the effect of hydration and polymer composition on the adhesion of immunoglobulin-G to poly(laurylmethacrylate-stat-glycerolmonomethacrylate-stat-ethylene-gly col-dimethacrylate) networks. Biomaterials 21:735–739. https://doi.org/10.1016/S0142-9612(99)00245-8
Lewis AL, Cumming ZL, Stratford PW (2001) Crosslinkable coatings from phosphorylcholine-based polymers. Biomaterials 22:99–111
Zhang ZR, Liu GJ, Bell S (2000) Synthesis of Poly(sol-ketal methacrylate)-block-poly(2-(dimethylamino)ethyl methacrylate) and preparation of nanospheres with cross-linked shells. Macromolecules 33:7877–7883. https://doi.org/10.1021/ma000781o
Li Z, Liu GJ (2003) Water-dispersible tetrablock copolymer synthesis, aggregation, nanotube preparation, and impregnation. Langmuir 19:10480–10486
Abdelaty MSA (2022) Comprehensive study of the phase transition temperature of poly (NIPAAm-co-DEAMCA-co-VA) terpolymers, post-serine and valine: thermal/pH and Hofmeister anions. Polym Bull. https://doi.org/10.1007/s00289-022-04337-5
Zhen Y, Wan S, Liu Y, Yan H, Shi R, Wang C (2005) Atom transfer radical polymerization of sol-ketal acrylate using cyclohexanone as the solvent. Macromol Chem Phys 206:607–612. https://doi.org/10.1002/macp.200400414
Feng X, Wu H, Sui X, Hempenius MA, JuliusVancso GV (2015) Thin film hydrogels from redox responsive poly(ferrocenylsilanes): preparation, properties, and applications in electrocatalysis. Eur Polym J 72:535–542. https://doi.org/10.1016/j.eurpolymj.2015.05.022
Tokarev I, Minko S (2009) Stimuli-responsive hydrogel thin films. Soft Matter 5:511–524. https://doi.org/10.1039/B813827C
Yang HW, Chena JK, Cheng CC, Kuo SW (2013) Association of poly(N-isopropylacrylamide) containing nucleobase multiple hydrogen bonding of adenine for DNA recognition. Appl Surf Sci 271:60–69. https://doi.org/10.1016/j.apsusc.2013.01.074
Gauthier MA, Gibson MI, Klok HA (2009) Synthesis of functional polymers by post-polymerization modification. Angew Chem Int Ed 48:48–58. https://doi.org/10.1002/anie.200801951
Fuchs AD, Tiller JC (2006) Contact-active antimicrobial coatings derived from aqueous suspensions. Angew Chem Int Ed 45:6759–6762. https://doi.org/10.1002/anie.200602738
Zolotukhin MG, Colquhoun HM, Sestiaa LG, Rueda DR, Flot D (2003) One-pot synthesis and characterization of soluble poly(aryl ether-ketone)s having pendant carboxyl groups. Macromolecule 36:4766–4771. https://doi.org/10.1021/ma0216977
Kanazawa H, Yamamoto K, Matsushima Y, Takai N, Kikuchi A, Sakurai Y, Okano T (1996) Temperature-responsive chromatography using poly(N-isopropylacrylamide)-modified silic. Anal Chem 68:100–105. https://doi.org/10.1021/ac950359j
Abdelaty MSA (2018) Poly(N-isopropylacrylamide-co-2-((diethylamino)methyl)-4-formyl-6-methoxyphenyl acrylate) environmental functional copolymers: synthesis, characterizations, and grafting with amino acids. Biomolecules 8:154. https://doi.org/10.3390/biom8040138
Abdelaty MSA (2018) Environmental functional photo-cross-linked hydrogel bilayer thin films from vanillin. J Polym Environ 26:2256. https://doi.org/10.1007/s10924-017-1126-y
Abdelaty MSA (2018) Environmental functional photo-cross-linked hydrogel bilayer thin films from vanillin (part 2): temperature-responsive layer A, functional, temperature and pH layer B. Polym Bull 11:4858. https://doi.org/10.1007/s00289-018-2297-y
Abdelaty MSA, Kuckling D (2016) Synthesis and characterization of new functional photo cross-linkable smart polymers containing vanillin derivatives. Gels 2:76. https://doi.org/10.3390/gels2010003
Kuckling D, Harmon ME, Frank CW (2002) A surface plasmon resonance study of volume phase transitions in N-Isopropylacrylamide gel films. Macromolecules 35:5999–6004. https://doi.org/10.1021/ma010985k
Harmon ME, Kuckling D, Frank CW (2003) Photo-cross-likable PNIPA copolymer 2: effects of constraint on temperature pH responsive hydrogel layers. Macromolecules 36:162–172. https://doi.org/10.1021/ma021025gCCC
Nan Zhang N, Knoll W (2009) Thermally responsive hydrogel films studied by surface plasmon diffraction. Anal Chem 81:2611-2617I. https://doi.org/10.1021/ac802527j
Aulasevich A, Junk MJN, Jakubowicz P, Roskamp RF, Menges B, Jonas U, Knoll W (2010) SPR/OWS angle spectra of (A) p-polarized and (B) s-polarized ligh. Macrmol Chem Phys 211:1018–1025. https://doi.org/10.1002/macp.200900533
Hosoya K, Kubo T, Tanaka N, Haginaka JA (2003) A possible purification method of DNAs’ fragments from humic matters in soil extracts using novel stimulus responsive polymer adsorbent. J Pharm Biomed Anal 30:1919–1922. https://doi.org/10.1016/S0731-7085(02)00535-6
Lu Y, Mei Y, Drechsler M, Ballauff M (2006) Thermosensitive core-shell particles as carriers for Ag nanoparticles: modulating the catalytic activity by a phase transition in networks. Angew Chem Int Ed 45:813–816. https://doi.org/10.1002/anie.200502731
Aminabhavi TM, Dharupaneedi SP (2017) 12—production of chitosan-based hydrogels for biomedical applications. In: Amber Jennings J, Bumgardner Joel D (eds) Chitosan based biomaterials Volume 1, Woodhead Publishing, pp. 295–319. https://doi.org/10.1016/B978-0-08-100230-8.00012-1.
Aminabhavi TM, Deshmukh AS (2016) Polysaccharide-based hydrogels as biomaterials. In: Kalia S (eds) Polymeric hydrogels as smart biomaterials. Springer series on polymer and composite materials. Springer, Cham. https://doi.org/10.1007/978-3-319-25322-0_3
Kip** M; PhD Thesis. Faculty of mathematics and science Technical univeristy Dressden (2008)
Thamizharasi S, Gnanasundaram P, Reddy BSR (1997) Copolymerization of 4-acetyl phenylacrylate with methyl methacrylate and butyl methacrylate: Synthesis, characterization and reactivity ratios. Eur Polymer J 33:1487–1494. https://doi.org/10.1016/S0014-3057(96)00270-4
Gupta S, Kuckling D, Kretschmer K, Choudhary V, Adler HJ (2007) Synthesis and characterization of stimuli-sensitive micro- and nanohydrogel based on photocrosslinkable poly(dimethylaminoethyl methacrylate). J Polym Sci Part A Polym Chem 45:669–679
Ahmed AA, Alan ET, Saad AK (2004) Solution rheology of hydrophobically modified associative polymers: effects of backbone composition and hydrophobe concentration. J Rheol 48:979–994. https://doi.org/10.1122/1.1773781
Acknowledgements
The author would like to express my gratitude to the chemistry department at the University of Paderborn.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdelaty, M.S.A., Abu-Zahra, N. Optimization of hydrophobic nonresponsive sol-ketal acrylate gel film to hydrophilic thermo-responsive gel by graft-polymerization. Polym. Bull. 81, 3169–3190 (2024). https://doi.org/10.1007/s00289-023-04847-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-023-04847-w