Abstract
Intercellular signaling and communication are used by bacteria to regulate a variety of behaviors. In a type of cell-cell communication known as quorum sensing (QS), which is mediated by a diffusible signaling molecule called an autoinducer, bacteria can undergo sudden changes in their behavior at a colony-wide level when the density of cells exceeds a critical threshold. In mathematical models of QS behavior, these changes can include the switch-like emergence of intracellular oscillations through a Hopf bifurcation, or sudden transitions between bistable steady-states as a result of a saddle-node bifurcation of equilibria. As an example of this latter type of QS transition, we formulate and analyze a cell-bulk ODE-PDE model in a 2-D spatial domain that incorporates the prototypical LuxI/LuxR QS system for a collection of stationary bacterial cells, as modeled by small circular disks of a common radius with a cell membrane that is permeable only to the autoinducer. By using the method of matched asymptotic expansions, it is shown that the steady-state solutions for the cell-bulk model exhibit a saddle-node bifurcation structure. The linear stability of these branches of equilibria are determined from the analysis of a nonlinear matrix eigenvalue problem, called the globally coupled eigenvalue problem. The key role on QS behavior of a bulk degradation of the autoinducer field, which arises from either a Robin boundary condition on the domain boundary or from a constant bulk decay, is highlighted. With bulk degradation, it is shown analytically that the effect of coupling identical bacterial cells to the bulk autoinducer diffusion field is to create an effective bifurcation parameter that depends on the population of the colony, the bulk diffusivity, the membrane permeabilities, and the cell radius. QS transitions occur when this effective parameter passes through a saddle-node bifurcation point of the Lux ODE kinetics for an isolated cell. In the limit of a large but finite bulk diffusivity, it is shown that the cell-bulk system is well-approximated by a simpler ODE-DAE system. This reduced system, which is used to study the effect of cell location on QS behavior, is easily implemented for a large number of cells. Predictions from the asymptotic theory for QS transitions between bistable states are favorably compared with full numerical solutions of the cell-bulk ODE-PDE system.

















Similar content being viewed by others
Availability of Data and Material
Not applicable
References
Abisado RG, Benomar S, Klaus JR, Dandekar AA, Chandler JR (2018) Bacterial quorum sensing and microbial community interactions. mBio 9(3):e02331-17
Alciatore DG, Miranda R (1995) A winding number and point-in-polygon algorithm. Glaxo Virtual Anatomy Project Research Report, Department of Mechanical Engineering, Colorado State University
Betcke T, Highan NG, Mehrmann V, Schröder C, Tisseur F (2013) NLEVP: A collection of nonlinear eigenvalue problems. ACM Trans Math Softw, 39(2):7.1–7.28
Chen W, Ward MJ (2011) The stability and dynamics of localized spot patterns in the two-dimensional Gray–Scott model. SIAM J Appl Dyn Syst 10(2):582–666
Danø S, Madsen MF, Sørensen PG (2007) Quantitative characterization of cell synchronization in yeast. Proc Natl Acad Sci 104(31):12732–12736
Danø S, Sørensen PG, Hynne F (1999) Sustained oscillations in living cells. Nature 402(6759):320–322
De Monte S, d’Ovidio F, Danø S, Sørensen PG (2007) Dynamical quorum sensing: population density encoded in cellular dynamics. Proc Natl Acad Sci 104(47):18377–18381
Dhooge A, Govaerts W, Kuznetsov YA, Meijer HGE, Sautois B (2008) New features of the software MatCont for bifurcation analysis of dynamical systems. Math Comput Model Dyn Syst 14(2):147–175
Dockery JD, Keener JP (2001) A mathematical model for quorum sensing in Pseudomonas aeruginosa. Bull Math Biol 63(1):95–116
Fagerlind MG, Rice SA, Nilsson P, Harlén M, James S, Charlton T, Kjelleberg S (2003) The role of regulators in the expression of quorum-sensing signals in Pseudomonas aeruginosa. J Mol Microbiol Biotechnol 6(2):88–100
Fan G, Bressloff P (2017) Population model of quorum sensing with multiple parallel pathways. Bull Math Bio 79(11):2599–2626
Fan G, Bressloff P (2019) Modeling the role of feedback in the adaptive response of bacterial quorum sensing. Bull Math Bio 81(5):1479–1505
FlexPDE. PDE Solutions inc (2015). Available at http://www.pdesolutions.com
Fuqua WC, Winans SC, Greenberg EP (1994) Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol 176(2):269–275
Goldbeter A (1996) Biochemical oscillations and cellular rhythms: the molecular bases of periodic and chaotic behaviour. Cambridge University press, Cambridge
Gou J, Ward MJ (2016) An asymptotic analysis of a 2-D model of dynamically active compartments coupled by bulk diffusion. J Nonlinear Sci 26(4):979–1029
Gregor T, Fujimoto K, Masaki N, Sawai S (2010) The onset of collective behavior in social amoebae. Science 328(5981):1021–1025
Güttel S, Tisseur F (2017) The nonlinear eigenvalue problem. Acta Numerica 26(1):1–94
Hou S-H (1998) Classroom note: a simple proof of the Leverrier–Faddeev characteristic polynomial algorithm. SIAM Rev 40(3):706–709
Iyaniwura S, Ward MJ (2020) Synchrony and oscillatory dynamics for a 2-D PDE-ODE model of diffusion-sensing with small signaling compartments. submitted, SIAM J Appl Dyn Sys (40 pages)
James S, Nilsson P, James G, Kjelleberg S, Fagerström T (2000) Luminescence control in the marine bacterium Vibrio fischeri: an analysis of the dynamics of lux regulation. J Mol Biol 296(4):1127–1137
Kaplan HB, Greenberg E (1985) Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J Bacteriol 163(3):1210–1214
Kato T (1995) Perturbation theory for linear operators, vol 132. Springer, Berlin, corrected printing of the second edition
Kolokolnikov T, Titcombe MS, Ward MJ (2005) Optimizing the fundamental Neumann eigenvalue for the Laplacian in a domain with small traps. Eur J Appl Math 16(2):161–200
Lohmiller W, Slotine JJ (1998) On contraction analysis for nonlinear systems. Automatica 34(6):683–696
Mandel P, Erneux T (1987) The slow passage through a steady bifurcation: delay and memory effects. J Stat Phys 48(5–6):1059–1070
Marenda M, Zanardo M, Trovato A, Seno F, Squartini A (2016) Modeling quorum sensing trade-offs between bacterial cell density and system extension from open boundaries. Sci Rep 6:39142
The Mathworks, Inc., Natick, Massachusetts. (2018) MATLAB version 9.4.0.813654 (R2018a)
Melke P, Sahlin P, Levchenko A, Jönsson H (2010) A cell-based model for quorum sensing in heterogeneous bacterial colonies. PLoS Comput Biol 6(6):e1000819
Miller MB, Bassler BL (2001) Quorum sensing in bacteria. Annu Rev Microbiol 55(1):165–199
Müller J, Kuttler C, Hense BA, Rothballer M, Hartmann A (2006) Cell-cell communication by quorum sensing and dimension-reduction. J Math Biol 53(4):672–702
Müller J, Uecker H (2013) Approximating the dynamics of communicating cells in a diffusive medium by ODEs - homogenization with localization. J Math Biol 67(5):1023–1065
Nanjundiah V (1998) Cyclic AMP oscillations in Dictyostelium discoideum: models and observations. Biophys Chem 72(1–2):1–8
Nealson KH, Platt T, Hastings JW (1970) Cellular control of the synthesis and activity of the bacterial luminescent system. J Bacteriol 104(1):313–322
Papenfort K, Bassler BL (2016) Quorum sensing signal-response systems in gram-negative bacteria. Nat Rev Microbiol 14(9):576–588
Pearson JP, Van Delden C, Iglewski BH (1999) Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J Bacteriol 181(4):1203–1210
Pérez-Velázquez J, Gölgeli M, García-Contreras R (2016) Mathematical modelling of bacterial quorum sensing: a review. Bull Math Biol 78(8):1585–1639
Ridgway W (2020) Analysis of a 2D model of quorum sensing characterized by a transition from bistability in spatially coupled signalling compartments. Master’s thesis, The University of British Columbia, Vancouver, Canada
Russo G, Slotine JJ (2010) Global convergence of quorum-sensing networks. Phys Rev E 82(4 Pt 1):041919
Trovato A, Seno F, Zanardo M, Alberghini S, Tondello A, Squartini A (2014) Quorum vs. diffusion sensing: a quantitative analysis of the relevance of absorbing or reflecting boundaries. FEMS Microbiol Lett 352(2):198–203
Van Delden C, Iglewski BH (1998) Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg Infect Dis 4(4):551–560
Waters CM, Bassler BL (2005) Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol 21:319–346
Williams JW, Cui X, Levchenko A, Stevens AM (2008) Robust and sensitive control of a quorum-sensing circuit by two interlocked feedback loops. Mol Syst Biol 4:234
Funding
Wesley Ridgway was supported by an NSERC CGS-M Fellowship as a graduate student at UBC. Michael Ward and Brian Wetton gratefully acknowledge the support of the NSERC Discovery Grant Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest/Competing Interests
Not applicable
Ethics approval
Not applicable
Consent to Participate
Not applicable
Consent for Publication
Not applicable. All authors are aware of the submission and approve.
Code availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendices
Non-Dimensionalization
We non-dimensionalize the cell-bulk model (1.1) and (1.2) and the Lux ODE system of Melke et al. (2010). Our dimensional model assumes units of concentration for the extracellular autoinducer and intracellular chemical species whereas the dimensional model in Gou and Ward (2016) uses both mass and concentration units. At the end of this appendix, we give the units for all of the quantities. In Table 1 we list the parameter values for parameter set P1 in Melke et al. (2010), along with their dimensionless counterparts given in (A.3).
We begin by non-dimensionalizing the Lux ODE kinetics for an isolated cell. In dimensional quantities and without bulk coupling, the system given in Melke et al. (2010) is
In our non-dimensionalization we eliminate as many parameters as possible, while ensuring that the ODE dynamics reaches its steady-state on an \({\mathcal {O}}(1)\) timescale. To this end, and with \(\mathbf {v}\equiv (v_1,\ldots ,v_4)^T\), we introduce the non-dimensional variables \(\mathbf {u}\) and t as
This choice eliminates \(\kappa _5\) and \(c_2\). New dimensionless ODE parameters are then defined as
By using (A.2) and (A.3) in (A.1), we obtain the dimensionless system for the reaction kinetics in (1.5).
The full ODE-PDE system is made dimensionless in a slightly different way than in Gou and Ward (2016). In (1.1) and (1.2) both \({\mathcal {U}}\) and \(\mathbf {v}_j\) have units of concentration (\(\text {moles}/\text {length}^2\)), while in Gou and Ward (2016), \(\mathbf {v}_j\) is measured in total amount (\(\text {moles}\)). With this in mind, we define the dimensionless quantities \(\mathbf {x}\) and \(U(\mathbf {x},t)\) by \(\mathbf {x}\equiv {\mathbf {X}/L}\) and \(U\equiv {{\mathcal {U}}/v_c}\). Upon substituting this into (1.1), we readily obtain (1.3) after defining the dimensionless bulk constants D, \(\gamma \), and \(\kappa \) and the dimensionless cell permeabilities \(d_{1j}\) and \(d_{2j}\) as
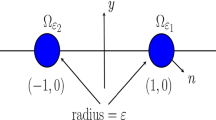
The requirement for the \(\varepsilon \)-dependent scaling in the permeabilities is so that there is an \({{\mathcal {O}}}(1)\) effect of the coupling of the cells to the bulk. Moreover, if \(\mathbf {X}\in \Omega _L\), where \(\Omega _L\) has a characteristic length scale of L, then \(\mathbf {x}\in \Omega _1\equiv \Omega \). The dimensionless kinetics in (1.4) follows from the definitions in (A.2) and (A.4).
Denoting \(\left[ x\right] \) to be the units of x, the units of the Lux and bulk parameters are as follows:

Green’s functions for the unit disk
To implement our steady-state and linear stability theory for the unit disk, two different Green’s functions are required. The Neumann Green’s function, satisfying, (3.5) is needed in §3 for the steady-state analysis with no bulk loss, and in § 5 to analyze the large \(D={{\mathcal {O}}}(\nu ^{-1})\) limiting regime. In the GCEP analysis in §3.2 for the \(D={{\mathcal {O}}}(1)\) regime, the eigenvalue-dependent Green’s function \(G_{\lambda }\) satisfying (3.17) is required. Setting \(\lambda =0\) in (3.17) yields the reduced-wave Green’s function in (3.11), which is required in § 3 for the steady-state analysis with bulk degradation.
In the unit disk, the Neumann Green’s function and its regular part are (see equation (4.3) of Kolokolnikov et al. (2005)):
Next, by extending the analysis in Appendix A.1 of Chen and Ward (2011) to allow for a Robin boundary condition, the Green’s function \(G_\lambda \) and its regular part \(R_\lambda \), satisfying (3.17), are calculated for the unit disk as
where \(\mathbf {x}=|\mathbf {x}|(\cos \phi ,\sin \phi )^T\) and \(\mathbf {x}_i=|\mathbf {x}_i|(\cos \phi _i,\sin \phi _i)^T\). Here \(\sigma _0 \equiv 1\), \(\sigma _n \equiv 2\) for \(n\ge 2\), and \(\gamma _e=0.57721\ldots \) is the Euler-Mascheroni constant. The functions \(K_n\) and \(I_n\) are the \(n^{\text {th}}\)-order modified Bessel functions of the first and second kind, respectively. Here, \(\theta _\lambda \equiv \sqrt{(\gamma + \lambda )/D}\), where the principle branch of the square root is taken when the argument is complex. Setting \(\lambda =0\) in (B.2) yields the result for the reduced-wave Green’s function and its regular part in (3.11).
When the centers \(\mathbf {x}_k\), for \(k=1,\ldots ,m\), of the cells are equally-spaced on a ring concentric within the unit disk, the Green’s matrices \({{\mathcal {G}}}_N\), \({{\mathcal {G}}}\), and \({{\mathcal {G}}}_{\lambda }\) as needed in the steady-state and linear stability analysis in § 3 are cyclic and symmetric matrices. As such, their matrix spectrum is available analytically.
For an \(m\times m\) cyclic matrix \({\mathcal {A}}\), with possibly complex-valued matrix entries, its complex-valued eigenvectors \(\tilde{\mathbf {v}}_j\) and eigenvalues \(\alpha _j\) are \(\alpha _j = \sum \nolimits _{k=1}^m {\mathcal {A}}_{1k} \omega _j^{k-1}\) and \(\tilde{\mathbf {v}}_j = \left( 1,\omega _j, ..., \omega _j^{m-1}\right) ^T\), for \(j = 1,\ldots , m\). Here \(\omega _j \equiv \exp {\left( \frac{2\pi i(j-1)}{m}\right) }\) and \({{\mathcal {A}}}_{1k}\), for \(k=1,\ldots ,m\), are the elements of the first row of \({{\mathcal {A}}}\). Since \({{\mathcal {A}}}\) is also a symmetric matrix, we have \({{\mathcal {A}}}_{1,j}={{\mathcal {A}}}_{1,m+2-j}\), for \(j=2,\ldots ,\lceil m/2\rceil \), where the ceiling function \(\lceil x \rceil \) is defined as the smallest integer not less than x. Consequently, \(\alpha _j=\alpha _{m+2-j}\), for \(j=2,\ldots ,\lceil m/2\rceil \), so that there are \(m-1\) eigenvalues with a multiplicity of two when m is odd, and \(m-2\) such eigenvalues when m is even. As a result, it follows that \(\frac{1}{2}\left[ \tilde{\mathbf {v}}_j+\tilde{\mathbf {v}}_{m+2-j}\right] \) and \(\frac{1}{2i}\left[ \tilde{\mathbf {v}}_j-\tilde{\mathbf {v}}_{m+2-j}\right] \) are two independent real-valued eigenvectors of \({{\mathcal {A}}}\), corresponding to the eigenvalues of multiplicity two. In this way, the matrix spectrum of a cyclic and symmetric matrix \({{\mathcal {A}}}\), with the normalized eigenvectors \(\mathbf {v}_j^T\mathbf {v}_j=1\), is
for \(j = 2,\ldots , \lceil m/2\rceil \), where \(\theta _j\equiv {2\pi (j-1)/m}\). When m is even, there is an additional normalized eigenvector of multiplicity one given by \(\mathbf {v}_{{m/2}+1}=m^{-1/2}(1,-1,1,\ldots ,-1)^T\).
Rights and permissions
About this article
Cite this article
Ridgway, W., Ward, M.J. & Wetton, B.T. Quorum-sensing induced transitions between bistable steady-states for a cell-bulk ODE-PDE model with lux intracellular kinetics. J. Math. Biol. 84, 5 (2022). https://doi.org/10.1007/s00285-021-01705-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00285-021-01705-z
Keywords
- Cell-bulk coupling
- Bulk diffusion
- Lux kinetics
- Quorum-sensing
- Bistable states
- Green’s function
- Globally coupled eigenvalue problem