Abstract
Oral mucositis is one of the worst effects of the conditioning regimens given to patients undergoing hematopoietic stem cell transplantation. It is characterized by dry mouth, erythema, mucosal soreness, ulcers, and pain, and it may impact patient outcomes. Bovine colostrum and Aloe vera contain a wide variety of biologically active compounds that promote mucosal healing. A non-randomized phase II study was designed to assess the safety and efficacy of a combined bovine colostrum and Aloe vera oral care protocol to prevent and to treat severe oral mucositis in transplant patients. Two commercially available products were given to patients in addition to the standard protocol: Remargin Colostrum OS® mouthwash and Remargin Colostrum Gastro-Gel® taken orally. Forty-six (78.0%) patients experienced oral mucositis, 40 (67.8%) developed mild–moderate forms, and 6 (10.2%) severe ones. Comparing the study group’s outcomes with those of a homogeneous historical control group, severe oral mucositis decreased significantly (10.2% vs. 28.4%; P < 0.01), as did its duration (0.5 ± 1.9 vs. 1.5 ± 3.0 days; P < 0.01). Febrile neutropenia episodes (69.5% vs. 95.1%; P < 0.01) and duration (4.0 ± 4.7 vs. 6.2 ± 4.5 days; P < 0.01) also decreased. These findings show that the experimental protocol seems effective in preventing severe forms of oral mucositis. However, a randomized controlled trial is necessary to confirm this.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Allogeneic and autologous hematopoietic stem cell transplantation (HSCT) are standards of treatment for several hematological malignancies [1,2,3]. Before stem cell infusion, the recipient is treated with a conditioning regimen that includes combinations of chemotherapy, radiotherapy, and/or immunotherapy [4, 5].
Chemotherapy- and/or radiotherapy-induced oral epithelial cell damage, known as oral mucositis (OM), is considered one of the worst toxic effects of conditioning regimens [6]. It is a predictable clinical condition favored also by some predisposing factors, including epigenetic, metabolomic, and microbiome-related ones [7,8,9], and it is experienced by the 70–100% of HSCT patients undergoing myeloablative conditioning regimens (MAC) [10,11,12,13,14]. Its signs and symptoms include dry mouth, taste and salivary change, erythema, mouth soreness, ulcers, and pain. Severe forms of OM (sOM) may impact patients’ quality of life (QoL) [15,16,17,18,19,20], as well as transplant-related morbidity and mortality, and healthcare costs [21,22,23,24].
The literature provides few evidence supporting strategies to prevent or treat OM [25, 26]. Thus, the approaches to dealing with OM in daily practice often rely on a wide variety of products supported by scarce or anecdotal evidence [27, 28]. An interest in using natural agents for OM has been increasing, since these products may be effective for symptom control and because their components can interfere with the pathobiological processes underlying OM development [29,30,31].
Bovine colostrum (BC) has a wide variety of biologically active components, including lactoferrin, lactoperoxidase, immunoglobulins, and growth factors, and its benefits on health as a dietary supplement have been widely studied [32,33,34]. The protective effects of BC on the intestinal mucosal barrier [35] and upper respiratory tract integrity [36,37,38] have been reported, as have its beneficial effects on boosting the immune system [39, 40]. In addition, topical applications of BC have been effective in both wound and mucosal healing thanks to its humectant, moisturizing, re-epithelizing, antioxidant, and immune-stimulant activities [41,42,43,44].
Aloe vera (AV)-based preparations contain various active compounds, including iron, folic acid, electrolytes, and vitamins, that have positive effects on general health [45,46,47,48]. Its formula shows emollient, moisturizing, anti-inflammatory, and immune-modulatory properties [49, 50], and has been studied for the prevention and treatment of several mucocutaneous conditions, without any adverse effects [51, 52].
Therefore, we hypothesized that combined formulas of BC and AV, in addition to the standard oral care practice, would effectively and safely prevent and treat sOM in patients undergoing HSCT.
Materials and methods
Study design and sample size
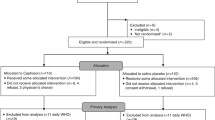
A single-arm, non-randomized, open label, single-center, phase II study was designed following the optimal two-stage design by Richard Simon [53]. Adult patients undergoing autologous or allogeneic HSCT were recruited; those who reported intolerance to the products’ components, who were not able to use the study self-reporting tools, or patients with OM already present at admission were excluded from the study. A study group (SG) sample size of 59 recipients was calculated assuming a reduction of 50% than local benchmarking data on sOM, and considering an α error of 0.05 and a sensitivity of 0.8. The study design provided a first step of 19 participants with a cutoff for study discontinuation of more than 5 patients with sOM. After recruitment, all patients received educational intervention (interview and educational material) on study medication management and the use of the tools included in the study. The study protocol was approved by the local ethics committee (n. 2016/0030535, December 28, 2016) and it was conducted in agreement with the Helsinki Declaration of 1975 and the Guidelines for Good Clinical Practice. All patients gave written informed consent before any study-related procedure took place.
Oral care protocol
In the transplant unit where the study has been performed, a standard oral care protocol was used to prevent and treat OM. It includes oral hygiene, i.e., gentle cleansing with toothbrush and toothpaste, followed by bland saline rinses (normal saline or sodium bicarbonate), 3 times per day after each meal, with the frequency increasing after OM onset. In addition, mouthwashes with moisturizing and emollient solutions and lip balm were recommended to all patients.
Two products containing BC and AV were added to standard practice in this study: (1) Remargin Colostrum OS® (RCOS), 10-ml single-dose stick pack natural mouthwashes containing water, Aloe barbadensis leaf juice, colostrum, glycerine, seed extracts, vegetable oils, sucralose, potassium sorbate, and citric acid (Solimè srl, Cavriago, Reggio Emilia, Italy, Patent No. 1291340); (2) Remargin Colostrum Gastro-Gel® (RCGG), 4-g single-dose stick-pack dietary supplement containing water, Aloe barbadensis gel, colostrum, maltodextrin, sorbitol, seed extracts, vegetable oils, sodium alginate, potassium sorbate, citric acid, and pectin (Solimè srl, Cavriago, Reggio Emilia, Italy, Patent No. 920596386).
Patients performed RCOS mouthwashes (1 stick pack) for 40–60 s after each oral hygiene, and RCGG (1 stick pack) was administered orally 3 times per day from the start of conditioning until OM onset (prevention phase). After OM onset (treatment phase), the frequency of intervention was increased to at least 3 to 5 times per day.
Endpoints and outcomes
The primary endpoint was the incidence of sOM (grade 3–4 WHO) during the study period. OM was assessed daily by the nursing staff starting from the first day of conditioning until day 21 post-transplant using the WHO scale. Secondary endpoints were the evaluation of overall OM incidence, its time of onset, and duration. OM-related pain scores were assessed daily using a 0–10 numerical rating scale (NRS). Neutropenia and febrile neutropenia (FN) duration (days) and FN events were recorded, as were some cost-related outcomes such as length of stay, antibiotic, antifungal and antiviral therapy, and days of opioid and parenteral nutrition. QoL was assessed weekly with EQ-5D-3L (not reported), and patient-reported data were collected using the Oral Mucositis Daily Questionnaire (OMDQ) (not reported). Adherence to the study protocol was monitored. Safety was assessed by collecting data on adverse events (AEs) and by monitoring blood cell count, hemoglobin, and serum levels of creatinine and bilirubin. Oral swab tests for infection were performed at admission and at day 8 post-transplant, while galactomannan serum levels were monitored weekly until day 28 post-transplant or discharge.
The study outcomes were compared with routinely collected local benchmarking data of a historical cohort of patients treated during the 22 months preceding the study start (Table 1). This cohort had undergone only the standard practice protocol to prevent and treat OM and was taken as the control group (CG). All the data were collected by the patients’ electronic clinical documentation and all the nurses assessing outcomes in both groups were routinely trained to use the assessment tools.
Descriptive analysis was performed using SPSS (IBM Corp. Released 2015, IBM SPSS Statistics for Windows, Version 23.0, Armonk, NY: IBM Corp.), and the Matrix Laboratory (MATLAB) Statistical toolbox version 2008 (MathWorks, Natick, MA, USA) was used for comparative analysis. All tests with P ≤ 0.05 were considered significant.
Results
Participants’ characteristics
Seventy-one HSCTs were performed from November 2017 to September 2019 in our hematology unit: 4 patients refused to participate in this study and 8 were ineligible. Thus, 59 patients were recruited 32 (54.2%) male and 27 (45.8%) female, with mean age 52.4 years (SD ± 12.0; range 18–71). All participants in the SG were adults and had no signs of OM at admission; 6 patients (10.2%) were smokers, 10 (16.9%) experienced OM during pre-transplant therapies, and none had a history of alcohol abuse. Most participants were married (42; 71.2%) and worked (44; 74.6%). Granulocyte-stimulating factor (GCSF) was administered to all autologous HSCT patients by day 1 post-transplant in both groups; only 1 allogeneic patient in SG was treated with GCSF from day + 18 due to infection. Main clinical information on SG and CG are showed in Table 1. No significant differences between groups are reported, including some risk factors for OM development.
Oral mucositis
During the first step of the study, 15/19 participants (78.9%) experienced OM; one patient (5.3%) developed sOM (grade 3 WHO), and no serious AEs were recorded in the report form. This made it possible to continue patient recruitment.
At the end of the study period (22 months), 46 (78.0%) patients experienced OM: 40 (67.8%) developed mild–moderate OM (WHO grades 1–2) and 6 (10.2%) developed sOM (WHO grades 3–4). Of those who developed sOM, 4 received allograft (2 BMF and 2 HL/NHL) and 2 patients had undergone autologous HSCT (2 PD). The incidence of patients without OM was 22.0% (13 cases). The mean duration of OM (any grade) was 7.9 days (SD ± 5.8); the mean duration of sOM was 0.5 days (SD ± 1.9). OM occurred on average 4.5 days (SD ± 2.4) post-transplant and 9.1 days (SD ± 3.5) after the start of conditioning regimen. The mean time of onset of sOM was 7.8 days (SD ± 1.7) post-transplant and 11.2 days (SD ± 2.9) from the first day of conditioning.
Historical cohort comparison
Severe OM incidence decreased more significantly in SG than in CG (10.2% vs. 28.4%, respectively; P < 0.01), while overall OM incidence remained unvaried (78.0% vs 80.2%; P = 0.74) (Table 2). No significant differences were seen in either overall or sOM time of onset with reference to HSCT and the start of conditioning. Severe OM mean duration was longer in CG (1.5 ± 3.0 vs. 0.5 ± 1.9 days; mean rank 75.9 vs. 63.1; P < 0.01), while there was no difference in overall duration of OM. Fewer patients in SG than in CG (41/59 vs. 77/81 patients, respectively; P < 0.01) developed FN, and its mean duration was shorter (4.0 ± 4.7 vs. 6.2 ± 4.5 days, respectively; P < 0.01) (Tables 2 and 3). No differences between the groups were found regarding neutropenia duration, length of hospital stay, maximum pain score (MPS), or the duration (days) of treatment with opioids, PN, antibiotics, or antifungal medications. The mean duration of antiviral therapy was significantly shorter in SG than in the CG (2.7 ± 7.3 vs. 9.5 ± 13.0; P < 0.01).
Adherence
Overall adherence to the study protocol is described in Table 4. During the prevention phase, 17/59 (28.8%) patients were fully compliant with the oral care protocol, while 42 (71.2%) did not take at least a dose of one of the experimental products. Thirty-five (59.3%) participants were 100% compliant with the RCOS mouthwashes; the mean percentage of adherence to prevention was 84.4 (SD ± 26.9). Seventeen patients (28.8%) were 100% compliant with RCGG administration; the mean percentage of adherence was 54.3 (SD ± 39.1). Forty-six patients developed OM. In the treatment phase, adherence was lower: 7/46 (15.2%) patients were compliant with at least 3 applications per day of the study protocol, while 39 (84.8%) were not. Twenty-four (52.2%) participants were 100% compliant with RCOS mouthwashes during this phase; the mean percentage of adherence was 77.8 (SD ± 34.7); 7 (15.2%) patients were 100% compliant with RCGG administration; the mean percentage of adherence was 28.3 (SD ± 39.1). The reasons for not complete adherence were primarily clinical (64.4% of prevention days; 63.3% of treatment days) due to chemotherapy-related gastrointestinal symptoms such as nausea and vomiting, diarrhea, and taste change. Voluntary adherence discontinuation due to fatigue symptoms was adopted in 34.6% of prevention days and 36.5% of treatment days, while intolerance to the study products was in 1.0% of prevention days and not specified in 0.2% of treatment days. In general, patients compliant with the intervention during treatment had significantly higher compliance during prevention (96.9% vs. 69.5%) and for RCGG (95.9% vs. 46.8%). During the prevention phase, no significant differences in adherence to RCOS and RCGG were found between patients who subsequently developed OM and those who did not (RCOS 83.8% vs. 85.4%: RCGG 54.3% vs. 53.9%) (Fig. 1). The development of sOM was correlated with lower adherence to RCGG during prevention (43.1% vs. 55.5%) and was associated with a decrease in adherence during the treatment phase (RCOS 52.1% vs. 70.2%; RCGG 16.7% vs. 22.5%) (Fig. 2). No differences in adherence to the study protocol were found per type of transplant or underlying disease.
Safety and adverse events
Of the 76 AEs recorded in the case report form, 14 (18.4%) occurred and resolved before OM onset (prevention phase), 28 (36.4%) were observed after OM onset (treatment phase), and 34 (44.7%) manifested across both periods. Most AEs (52; 68.4%) were conditioning-related gastrointestinal symptoms (Fig. 3). No deviations of the monitored biochemical parameters were detected during and after the study period, and none of the registered AEs resulted correlated with the experimental oral care protocol. Weekly monitoring of galactomannan serum levels did not show alterations. At admission, 22 (37.3%) of SG patients’ oral cavities were colonized by Candida albicans, 36 (61.0%) were negative and 1 (1.7%) was colonized by Streptococcus agalactiae. On day 8 post-transplant, candida-colonized mouths decreased to 18 (30.5%), while 40 (67.8%) were negative and 1 (1.7%) was positive to Saccharomyces cerevisiae.
Discussion
The safety and efficacy of BC- and AV-based oral care protocol on HSCT patients with sOM were assessed in our prospective study. Safety was monitored by analyzing data on AEs, biochemical parameters, and culture tests routinely collected during the patients’ hospital stay. The compared groups were homogeneous in terms of number of participants, sex, age, type of transplant, underlying diagnosis, stem cell source, type of cell product, conditioning regimen, immunosuppressive strategy, growth factor use, and risk factors for OM development. Despite there being no difference between groups in overall OM development, a reduction of up to 60% of sOM incidence was found in SG. In addition, sOM mean duration per group appeared shorter in SG, and a correlation between the reduction in sOM incidence and the reduction in the number of FN episodes and duration appeared evident. Rathe et al. (2020) [44] explored the effects on chemotherapy-related toxicities of daily BC dietary supplementation obtaining a reduction of OM peak of severity in the treatment group more than in the control group. However, in Rathe’s trial, as OM severity was a secondary endpoint, the risk of an underpowered sample was posed. Furthermore, the effects of BC supplementation on FN were not significant, while our study suggested a reduction effect.
Beneficial effects of bioactive milk factors on oral mucosa exposed to chemotherapy were described in a pre-clinical study on hamster [54]. Significant reduction in severity and duration of OM was reported in two studies on HSCT patients undergoing chemotherapy-based conditioning regimens where whey proteins were administered as dietary supplements (systemic effect) [55] and as mouthwashes (topical effect) [56].
BC antibacterial activity conferred by lactoferrin, lactoperoxidase, and a variety of immunoglobulins [57,58,59], combined with the antimicrobial properties of AV against the Candida species [60,61,62] and type 2 herpes simplex [63], could explain some of our significant findings, such as the reduction in FN (episodes and duration) and the reduced use of antiviral medication in SG. Weak evidences of the benefits of BC on the integrity of the mucosal barrier, reducing intestinal bacteria translocation, have been reported [33, 34], and AV’s in vitro antiviral action has been described [63]. The immunomodulatory and anti-inflammatory effects of AV were provided by the stimulation of macrophages and modulating cyclooxygenase activation pathway [47, 63,64,65], which is fundamental to OM pathobiology [11]. BC contains a range of cytokines and other non-antimicrobial substances that together modulate inflammation and maintain or improve host response under different immune system exposures [40, 66].
Dietary supplementation of BC may trigger immunological events that lead to systemic effects [33]. However, the limited patient adherence to RCGG intake gave rise to several doubts on the real potency of any systemic effect in this study.
The beneficial effects of AV and of BC on wound care have been suggested by some preclinical studies [41, 67, 68]. Their effects on mucocutaneous issues such as pain reduction, wound healing acceleration, stomatitis healing, and QoL improvement are well known [42, 43, 51, 52]. It has been suggested that some components of BC, such as nucleotides, epidermal growth factor (EGF), and insulin-like growth factor-1 (IGF-1), promote mucocutaneous cellular growth and also help repair gene impairment [32]. The tissue regenerative properties of AV are due to its component mannose-6-phosphate (M6P), which plays a fundamental role in extracellular matrix remodelling as well as in increasing proliferation of fibroblasts and collagen and in producing some fundamental substances such as hyaluronic acid [69,70,71].
In our study, the anti-inflammatory and antimicrobial properties and the tissue healing capability of AV and BC described above were beneficial during the ulcerative phase of OM, when the oral microbial flora plays a fundamental role in amplifying gene signals, accelerating tissue damage, and increasing inflammation, pain, and the risk of systemic infections [11]. Therefore, the effects of the oral care protocol were observed primarily on sOM development and duration. Although not statistically significant, the observed reduction in the number of days of opioids use in SG might confirm this hypothesis.
The reduced compliance to the oral care protocol due to factors such as chemotherapy-related toxicities may be a limiting factor of this study. Patients affected by nausea, vomiting, and/or taste change had difficulties taking study products due to their consistence (RCGG) and/or flavor (RCOS). In particular, difficulties in RCGG swallowing were reported by these patients, especially after mucositis onset. Although our results suggest a strong effect, the oral care protocol included different strategies, such as topical and systemic interventions, which precluded any consideration on the effect of the individual products. The comparison with the historical control group may have involved the change in some undetectable variables, leading to result biases. Furthermore, the strategies for supportive therapy and care may have varied because of healthcare professionals’ decisions or patients’ needs.
To our knowledge, this was the first study on the combination of BC and AV for the prevention and treatment of OM in oncology setting. The oral care protocol investigated in this study showed significant results on sOM incidence without any significant AEs. Our findings may be explained by the activity of the multiple bioactive substances composing BC and AV, and secondary findings seem to confirm the antimicrobial effects of both compounds, as already suggested in the literature [33, 34, 47, 60]. However, the study design and some limitations suggest caution when interpreting these results. A randomized controlled trial is necessary to provide evidence in favor or against the use of this approach in clinical practice. It is implemented at our institute.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Balassa K, Danby R, Rocha V (2019) Haematopoietic stem cell transplants: principles and indications. Br J Hosp Med (Lond) 80(1):33–39. https://doi.org/10.12968/hmed.2019.80.1.33
Maziarz R (2015) Overview of hematopoietic stem cell transplantation. In: Maziarz R, Slater S (eds) Blood and marrow transplant handbook Springer Cham https://doi.org/10.1007/978-3-319-13832-9_1
Gratwohl A, Baldomero H, Aljurf M, Pasquini MC, Bouzas LF, Yoshimi A, Szer J, Lipton J, Schwendener A, Gratwohl M, Frauendorfer K, Niederwieser D, Horowitz M, Kodera Y (2010) Worldwide Network of Blood and Marrow Transplantation Hematopoietic stem cell transplantation: a global perspective. JAMA 28(30316):1617–24. https://doi.org/10.1001/jama.2010.491
Copelan EA (2006) Hematopoietic stem-cell transplantation. N Engl J Med 27(354 17):1813–26. https://doi.org/10.1056/NEJMra052638
Zulu S, Kenyon M (2018) Principles of conditioning therapy and cell infusion. In: Kenyon M, Babic A (eds) The European blood and marrow transplantation textbook for nurses: under the auspices of EBMT [Internet] Cham (CH): Springer Chapter 6
Bellm LA, Epstein JB, Rose-Ped A, Martin P, Fuchs HJ (2000) Patient reports of complications of bone marrow transplantation. Support Care Cancer 8(1):33–39. https://doi.org/10.1007/s005209900095
Sonis ST (2009) Mucositis: the impact, biology and therapeutic opportunities of oral mucositis. Oral Oncol 45(12):1015–1020. https://doi.org/10.1016/j.oraloncology.2009.08.006
Bachour PC, Sonis ST (2018) Predicting mucositis risk associated with cytotoxic cancer treatment regimens: rationale, complexity, and challenges. Curr Opin Support Palliat Care 12(2):198–210. https://doi.org/10.1097/SPC.0000000000000339
Sonis ST (2021) Treatment for oral mucositis-current options and an update of small molecules under development. Curr Treat Options Oncol 17(22 3):25. https://doi.org/10.1007/s11864-021-00823-6
Filicko J, Lazarus HM, Flomenberg N (2003) Mucosal injury in patients undergoing hematopoietic progenitor cell transplantation: new approaches to prophylaxis and treatment. Bone Marrow Transplant 31(1):1–10. https://doi.org/10.1038/sj.bmt.1703776
Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, Bekele BN, Raber-Durlacher J, Donnelly JP, Rubenstein EB; Mucositis Study Section of the Multinational Association for Supportive Care in Cancer; International Society for Oral Oncology (2004) Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 100(9 Suppl):1995–2025. https://doi.org/10.1002/cncr.20162
Cawley MM, Benson LM (2005) Current trends in managing oral mucositis. Clin J Oncol Nurs 9(5):584–592. https://doi.org/10.1188/05.CJON.584-592
Chaudhry HM, Bruce AJ, Wolf RC, Litzow MR, Hogan WJ, Patnaik MS, Kremers WK, Phillips GL, Hashmi SK (2016) The incidence and severity of oral mucositis among allogeneic hematopoietic stem cell transplantation patients: a systematic review. Biol Blood Marrow Transplant 22(4):605–616. https://doi.org/10.1016/j.bbmt.2015.09.014
Vagliano L, Feraut C, Gobetto G, Trunfio A, Errico A, Campani V, Costazza G, Mega A, Matozzo V, Berni M, Alberani F, Banfi MM, Martinelli L, Munaron S, Orlando L, Lubiato L, Leanza S, Guerrato R, Rossetti A, Messina M, Barzetti L, Satta G, Dimonte V (2011) Incidence and severity of oral mucositis in patients undergoing haematopoietic SCT–results of a multicentre study. Bone Marrow Transplant 46(5):727–732. https://doi.org/10.1038/bmt.2010.184
Yüce UÖ, Yurtsever S (2019) Effect of education about oral mucositis given to the cancer patients having chemotherapy on life quality. J Cancer Educ 34(1):35–40. https://doi.org/10.1007/s13187-017-1262-z
Blijlevens N, Sonis S (2007) Palifermin (recombinant keratinocyte growth factor-1): a pleiotropic growth factor with multiple biological activities in preventing chemotherapy- and radiotherapy-induced mucositis. Ann Oncol 18(5):817–826. https://doi.org/10.1093/annonc/mdl332
Treister NS, Sankar V (2022) Chemotherapy-induced oral mucositis. Medscape. Available at: https://emedicine.medscape.com/article/1079570-overview?reg=1#a1. Accessed 1 April 2022.
Rose-Ped AM, Bellm LA, Epstein JB, Trotti A, Gwede C, Fuchs HJ (2002) Complications of radiation therapy for head and neck cancers: the patient’s perspective. Cancer Nurs 25(6):461–467. https://doi.org/10.1097/00002820-200212000-00010
Dodd MJ, Dibble S, Miaskowski C, Paul S, Cho M, MacPhail L, Greenspan D, Shiba G (2001) A comparison of the affective state and quality of life of chemotherapy patients who do and do not develop chemotherapy-induced oral mucositis. J Pain Symptom Manage 21(6):498–505. https://doi.org/10.1016/s0885-3924(01)00277-9
Borbasi S, Cameron K, Quested B, Olver I, To B, Evans D (2002) More than a sore mouth: patients’ experience of oral mucositis. Oncol Nurs Forum 29(7):1051–1057. https://doi.org/10.1188/02.ONF.1051-1057
Vera-Llonch M, Oster G, Ford CM, Lu J, Sonis S (2007) Oral mucositis and outcomes of allogeneic hematopoietic stem-cell transplantation in patients with hematologic malignancies. Support Care Cancer 15(5):491–496. https://doi.org/10.1007/s00520-006-0176-9
Sonis ST, Oster G, Fuchs H, Bellm L, Bradford WZ, Edelsberg J, Hayden V, Eilers J, Epstein JB, LeVeque FG, Miller C, Peterson DE, Schubert MM, Spijkervet FK, Horowitz M (2001) Oral mucositis and the clinical and economic outcomes of hematopoietic stem-cell transplantation. J Clin Oncol 19(8):2201–2205. https://doi.org/10.1200/JCO.2001.19.8.2201
Rodrigues-Oliveira L, Kowalski LP, Santos M, Marta GN, Bensadoun RJ, Martins MD, Lopes MA, Castro G Jr, William WN Jr, Chaves ALF, Migliorati CA, Salloum RG, Rodrigues-Fernandes CI, Kauark-Fontes E, Brandão TB, Santos-Silva AR, Prado-Ribeiro AC (2021) Direct costs associated with the management of mucositis: a systematic review. Oral Oncol 118:105296. https://doi.org/10.1016/j.oraloncology.2021.105296
van Leeuwen SJM, Proctor GB, Laheij AMGA, Potting CMJ, Smits O, Bronkhorst EM, Hazenberg MD, Haverman TM, Brennan MT, von Bültzingslöwen I, Raber-Durlacher JE, Huysmans MCDNJM, Rozema FR, Blijlevens NMA (2021) Significant salivary changes in relation to oral mucositis following autologous hematopoietic stem cell transplantation. Bone Marrow Transplant 56(6):1381–1390. https://doi.org/10.1038/s41409-020-01185-7
Miller AB, Hoogstraten B, Staquet M, Winkler A (1981) Reporting results of cancer treatment. Cancer 47(1):207–214. https://doi.org/10.1002/1097-0142(19810101)47:1%3c207::aid-cncr2820470134%3e3.0.co;2-6
Elad S, Cheng KKF, Lalla RV, Yarom N, Hong C, Logan RM, Bowen J, Gibson R, Saunders DP, Zadik Y, Ariyawardana A, Correa ME, Ranna V, Bossi P; Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO) (2020) MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 126(19):4423–4431. https://doi.org/10.1002/cncr.33100
Miranda-Silva W, Gomes-Silva W, Zadik Y, Yarom N, Al-Azri AR, Hong CHL, Ariyawardana A, Saunders DP, Correa ME, Arany PR, Bowen J, Cheng KKF, Tissing WJE, Bossi P, Elad S; Mucositis Study Group of the Multinational Association of Supportive Care in Cancer / International Society for Oral Oncology (MASCC/ISOO) (2021) MASCC/ISOO clinical practice guidelines for the management of mucositis: sub-analysis of current interventions for the management of oral mucositis in pediatric cancer patients. Support Care Cancer 29(7):3539–3562. https://doi.org/10.1007/s00520-020-05803-4
Mawardi H, Treister N, Felemban O, Alamoudi W, Algohary G, Alsultan A, Alshehri N, Tazi I, Shaheen M, Alsharani M, Alshemmari S, Arat M, Bekadja MA, Al-Khabori M, Okaily S, Ali N, Abujazar H, Jastaniah W, Hamidieh AA, Hashmi S, Aljurf M (2021) Current practice of oral care for hematopoietic stem cell transplant patients: a survey of the Eastern Mediterranean Blood and Marrow transplantation group. Hematol Oncol Stem Cell Ther S1658–3876(21):00006–6. https://doi.org/10.1016/j.hemonc.2021.01.006
Sonis ST (2011) Oral mucositis. Anticancer Drugs 22(7):607–612. https://doi.org/10.1097/CAD.0b013e3283462086
Yarom N, Hovan A, Bossi P, Ariyawardana A, Jensen SB, Gobbo M, Saca-Hazboun H, Kandwal A, Majorana A, Ottaviani G, Pentenero M, Nasr NM, Rouleau T, Lucas AS, Treister NS, Zur E, Ranna V, Vaddi A, Barasch A, Lalla RV, Cheng KKF, Elad S; Mucositis Study Group of the Multinational Association of Supportive Care in Cancer / International Society of Oral Oncology (MASCC/ISOO) (2020) Systematic review of natural and miscellaneous agents, for the management of oral mucositis in cancer patients and clinical practice guidelines - part 2: honey, herbal compounds, saliva stimulants, probiotics, and miscellaneous agents. Support Care Cancer 28(5):2457–2472. https://doi.org/10.1007/s00520-019-05256-4
Yarom N, Hovan A, Bossi P, Ariyawardana A, Jensen SB, Gobbo M, Saca-Hazboun H, Kandwal A, Majorana A, Ottaviani G, Pentenero M, Nasr NM, Rouleau T, Lucas AS, Treister NS, Zur E, Ranna V, Vaddi A, Cheng KKF, Barasch A, Lalla RV, Elad S; Mucositis Study Group of the Multinational Association of Supportive Care in Cancer / International Society of Oral Oncology (MASCC/ISOO) (2019) Systematic review of natural and miscellaneous agents for the management of oral mucositis in cancer patients and clinical practice guidelines-part 1: vitamins, minerals, and nutritional supplements. Support Care Cancer 27(10):3997–4010. https://doi.org/10.1007/s00520-019-04887-x
McGrath BA, Fox PF, McSweeney PLH, Kelly AL (2016) Composition and properties of bovine colostrum: a review. Dairy Sci & Technol 96(2):133–158. https://doi.org/10.1007/s13594-015-0258-x
Rathe M, Müller K, Sangild PT, Husby S (2014) Clinical applications of bovine colostrum therapy: a systematic review. Nutr Rev 72(4):237–254. https://doi.org/10.1111/nure.12089
Guberti M, Botti S, Capuzzo MT, Nardozi S, Fusco A, Cera A, Dugo L, Piredda M, De Marinis MG (2021) Bovine colostrum applications in sick and healthy people: a systematic review. Nutrients 13(7):2194. https://doi.org/10.3390/nu13072194
Menchetti L, Traina G, Tomasello G, Casagrande-Proietti P, Leonardi L, Barbato O, Brecchia G (2016) Potential benefits of colostrum in gastrointestinal diseases. Front Biosci (Schol Ed) 8:331–351. https://doi.org/10.2741/s467
Jones AW, Cameron SJS, Thatcher R, Beecroft MS, Mur LAJ, Davison G (2014) Effects of bovine colostrum supplementation on upper respiratory illness in active males. Brain Behav Immun 39:194–203. https://doi.org/10.1016/j.bbi.2013.10.032
Saad K, Abo-Elela MGM, El-Baseer KAA, Ahmed AE, Ahmad FA, Tawfeek MSK, El-Houfey AA, Aboul Khair MD, Abdel-Salam AM, Abo-Elgheit A, Qubaisy H, Ali AM, Abdel-Mawgoud E (2016) Effects of bovine colostrum on recurrent respiratory tract infections and diarrhea in children. Medicine (Baltimore) 95(37):e4560. https://doi.org/10.1097/MD.0000000000004560
Patıroğlu T, Kondolot M (2013) The effect of bovine colostrum on viral upper respiratory tract infections in children with immunoglobulin A deficiency. Clin Respir J 7(1):21–26. https://doi.org/10.1111/j.1752-699X.2011.00268.x
Dice Nail C (2016) The immunoprotective properties of bovine colostrum: a review. Nutritional Perspectives: Journal of the Council on Nutrition 39(3):23–30. Available from: https://search.ebscohost.com/login.aspx?direct=true&db=ccm&AN=117004037&site=ehost-live. Accessed 3 February 2022.
Gopal PK, Gill HS (2000) Oligosaccharides and glycoconjugates in bovine milk and colostrum. Br J Nutr 84(Suppl 1):S69-74. https://doi.org/10.1017/s0007114500002270
Doillon CJ, Lehance F, Bordeleau LJ, Laplante-Campbell MP, Drouin R (2011) Modulatory effect of a complex fraction derived from colostrum on fibroblast contractibility and consequences on repair tissue. Int Wound J 8(3):280–290. https://doi.org/10.1111/j.1742-481X.2011.00783.x
Nappi RE, Benedetto C, Campolo F, Martella S, Tosti C, Cianci A, Caruso S, Guaschino S, Grimaldi E, Bagolan M, Sardina M (2016) Efficacy, tolerability and safety of a new medical device, Monurelle Biogel(®) vaginal gel in the treatment of vaginal dryness: a randomized clinical trial in women of reproductive age. Eur J Obstet Gynecol Reprod Biol 203:82–88. https://doi.org/10.1016/j.ejogrb.2016.05.005
Schiavi MC, Di Tucci C, Colagiovanni V, Faiano P, Giannini A, D’Oria O, Prata G, Perniola G, Monti M, Zullo MA, Muzii L, Benedetti Panici P (2019) A medical device containing purified bovine colostrum (Monurelle Biogel) in the treatment of vulvovaginal atrophy in postmenopausal women: retrospective analysis of urinary symptoms, sexual function, and quality of life. Low Urin Tract Symptoms 11(2):O11–O15. https://doi.org/10.1111/luts.12204
Rathe M, De Pietri S, Wehner PS, Frandsen TL, Grell K, Schmiegelow K, Sangild PT, Husby S, Müller K (2020) Bovine colostrum against chemotherapy-induced gastrointestinal toxicity in children with acute lymphoblastic leukemia: a randomized, double-blind, placebo-controlled trial. JPEN J Parenter Enteral Nutr 44(2):337–347. https://doi.org/10.1002/jpen.1528
El-Gammal A, Nardo VD, Daaboul F, Tchernev G, Wollina U, Lotti J, Lotti T (2018) Is there a place for local natural treatment of psoriasis? Open Access Maced J Med Sci 6(5):839–842. https://doi.org/10.3889/oamjms.2018.106
Panahi Y, Davoudi SM, Sahebkar A, Beiraghdar F, Dadjo Y, Feizi I, Amirchoopani G, Zamani A (2012) Efficacy of Aloe vera/olive oil cream versus betamethasone cream for chronic skin lesions following sulfur mustard exposure: a randomized double-blind clinical trial. Cutan Ocul Toxicol 31(2):95–103. https://doi.org/10.3109/15569527.2011.614669
Radha MH, Laxmipriya NP (2014) Evaluation of biological properties and clinical effectiveness of Aloe vera: a systematic review. J Tradit Complement Med 5(1):21–26. https://doi.org/10.1016/j.jtcme.2014.10.006
Tanaka M, Yamamoto Y, Misawa E, Nabeshima K, Saito M, Yamauchi K, Abe F, Furukawa F (2016) Aloe sterol supplementation improves skin elasticity in Japanese men with sunlight-exposed skin: a 12-week double-blind, randomized controlled trial. Clin Cosmet Investig Dermatol 9:435–442. https://doi.org/10.2147/CCID.S118947
Choonhakarn C, Busaracome P, Sripanidkulchai B, Sarakarn P (2008) The efficacy of Aloe vera gel in the treatment of oral lichen planus: a randomized controlled trial. Br J Dermatol 158(3):573–577. https://doi.org/10.1111/j.1365-2133.2007.08370.x
Choonhakarn C, Busaracome P, Sripanidkulchai B, Sarakarn P (2010) A prospective randomized clinical trial comparing topical Aloe vera with 0.1% triamcinolone acetonide in mild to moderate plaque psoriasis. J Eur Acad Dermatol Venereol 24(2):168–72. https://doi.org/10.1111/j.1468-3083.2009.03377.x
Gok Metin Z, Helvaci A, Gulbahar Eren M (2021) Effects of Aloe vera in adults with mucocutaneous problems: a systematic review and meta-analysis. J Adv Nurs 77(3):1105–1126. https://doi.org/10.1111/jan.14653
da Lima ICGS, de SoutoMaior LF, Gueiros LAM, Leão JC, Higino JS, Carvalho AAT (2021) Clinical applicability of natural products for prevention and treatment of oral mucositis: a systematic review and meta-analysis. Clin Oral Investig 25(6):4115–4124. https://doi.org/10.1007/s00784-020-03743-1
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10(1):1–10. https://doi.org/10.1016/0197-2456(89)90015-9
Clarke J, Butler R, Howarth G, Read L, Regester G (2002) Exposure of oral mucosa to bioactive milk factors reduces severity of chemotherapy-induced mucositis in the hamster. Oral Oncol 38(5):478–485. https://doi.org/10.1016/s1368-8375(01)00107-5
Perrone AC, Barbosa TR, da Silva FL, Perrone ÍT, de Carvalho AF, Stephani R, Dos Santos KB, Atalla Â, Hallack Neto AE (2017) Supplementation with concentrated milk protein in patients undergoing hematopoietic stem cell transplantation. Nutrition 37:1–6. https://doi.org/10.1016/j.nut.2016.10.010
Prince HM, Regester G, Gates P, Jablonskis L, Seymour JF, Lillie K, West R, Wolf M, Januszewicz H, Belford D (2005) A phase Ib clinical trial of PV701, a milk-derived protein extract, for the prevention and treatment of oral mucositis in patients undergoing high-dose BEAM chemotherapy. Biol Blood Marrow Transplant 11(7):512–520. https://doi.org/10.1016/j.bbmt.2005.04.001
Barrington GM, Besser TE, Davis WC, Gay CC, Reeves JJ, McFadden TB (1997) Expression of immunoglobulin G1 receptors by bovine mammary epithelial cells and mammary leukocytes. J Dairy Sci 80(1):86–93. https://doi.org/10.3168/jds.S0022-0302(97)75915-0
Butler JE (1973) The occurrence of immunoglobulin fragments two types of lactoferrin and a lactoferrin-IgG2 complex in bovine colostral and milk whey Biochimica et Biophysica Acta (BBA) -. Protein Structure 295(1):341–351. https://doi.org/10.1016/0005-2795(73)90101-3
Pakkanen R, Aalto J (1997) Growth factors and antimicrobial factors of bovine colostrum. Int Dairy J 7(5):285–297. https://doi.org/10.1016/S0958-6946(97)00022-8
Cataldi V, Di Bartolomeo S, Di Campli E, Nostro A, Cellini L, Di Giulio M (2015) In vitro activity of Aloe vera inner gel against microorganisms grown in planktonic and sessile phases. Int J Immunopathol Pharmacol 28(4):595–602. https://doi.org/10.1177/0394632015600594
Bernardes I, Felipe Rodrigues MP, Bacelli GK, Munin E, Alves LP, Costa MS (2012) Aloe vera extract reduces both growth and germ tube formation by Candida albicans. Mycoses 55(3):257–261. https://doi.org/10.1111/j.1439-0507.2011.02079.x
Das S, Mishra B, Gill K, Ashraf MS, Singh AK, Sinha M, Sharma S, Xess I, Dalal K, Singh TP, Dey S (2011) Isolation and characterization of novel protein with anti-fungal and anti-inflammatory properties from Aloe vera leaf gel. Int J Biol Macromol 48(1):38–43. https://doi.org/10.1016/j.ijbiomac.2010.09.010
Zandi K, Zadeh MA, Sartavi K, Rastian Z (2007) Antiviral activity of Aloe vera against herpes simplex virus type 2: an in vitro study. Afri J Biotechnol 6(15) https://doi.org/10.5897/AJB2007.000-2276
Salehi B, Lopez-Jornet P, Pons-Fuster López E, Calina D, Sharifi-Rad M, Ramírez-Alarcón K, Forman K, Fernández M, Martorell M, Setzer WN, Martins N, Rodrigues CF, Sharifi-Rad J (2019) Plant-derived bioactives in oral mucosal lesions: a key emphasis to curcumin, lycopene, chamomile, Aloe vera, green tea and coffee properties. Biomolecules 9(3):106. https://doi.org/10.3390/biom9030106
Vijayalakshmi D, Dhandapani R, Jayaveni S, Jithendra PS, Rose C, Mandal AB (2012) In vitro anti inflammatory activity of Aloe vera by down regulation of MMP-9 in peripheral blood mononuclear cells. J Ethnopharmacol 141(1):542–546. https://doi.org/10.1016/j.jep.2012.02.040
Sun Q, Chen X, Yu J, Zen K, Zhang CY, Li L (2013) Immune modulatory function of abundant immune-related microRNAs in microvesicles from bovine colostrum. Protein Cell 4(3):197–210. https://doi.org/10.1007/s13238-013-2119-9
Barrantes E, Guinea M (2003) Inhibition of collagenase and metalloproteinases by aloins and aloe gel. Life Sci 72(7):843–850. https://doi.org/10.1016/s0024-3205(02)02308-1
Wynn RL (2005) Aloe vera gel: Update for dentistry. Gen Dent 53(1):6–9
Jettanacheawchankit S, Sasithanasate S, Sangvanich P, Banlunara W, Thunyakitpisal P (2009) Acemannan stimulates gingival fibroblast proliferation; expressions of keratinocyte growth factor-1, vascular endothelial growth factor, and type I collagen; and wound healing. J Pharmacol Sci 109(4):525–531. https://doi.org/10.1254/jphs.08204fp
Tabandeh MR, Oryan A, Mohammadalipour A (2014) Polysaccharides of Aloe vera induce MMP-3 and TIMP-2 gene expression during the skin wound repair of rat. Int J Biol Macromol 65:424–430. https://doi.org/10.1016/j.ijbiomac.2014.01.055
Takahashi M, Kitamoto D, Asikin Y, Takara K, Wada K (2009) Liposomes encapsulating Aloe vera leaf gel extract significantly enhance proliferation and collagen synthesis in human skin cell lines. J Oleo Sci 58(12):643–650. https://doi.org/10.5650/jos.58.643
Acknowledgements
The authors would like to thank to Dr. Roberto Solimè for providing us with the products without expecting anything in exchange. Many thanks to Jacqueline M. Costa for the English-language editing. This study was partially supported by Italian Ministry of Health - Ricerca Corrente Annual Program 2023.
Author information
Authors and Affiliations
Contributions
Study conceptualization and methodology: Monica Guberti; data collection, study management, and paper draft writing: Stefano Botti; data collection and participants’ recruitment: Cristiana Caffarri; data analysis and table/figure preparation: Silvio Cavuto, Luisa Savoldi; data management and case report form management: Andrea Fusco; study conceptualization, data curation, and results interpretation: Francesco Merli; paper writing, editing, and study supervision: Michela Piredda; project supervision and administration: Maria Grazia De Marinis. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved (n. 2016/0030535, December 28, 2016) by the Area Vasta Emilia Nord (AVEN) ethics committee and it was conducted in agreement with the Helsinki Declaration of 1975 and the Guidelines for Good Clinical Practice. All participants gave written informed consent before any study-related procedure took place.
Competing interests
The authors declare no competing interests.
Consent for publication
All participants provided written consent to data management and publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guberti, M., Botti, S., Caffarri, C. et al. Efficacy and safety of a colostrum- and Aloe vera-based oral care protocol to prevent and treat severe oral mucositis in patients undergoing hematopoietic stem cell transplantation: a single-arm phase II study. Ann Hematol 101, 2325–2336 (2022). https://doi.org/10.1007/s00277-022-04934-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-022-04934-4