Abstract
Magnetic and structure transitions of Mn3–xFexO4 solid solutions under extreme conditions are clarified by neutron time-of-flight scattering diffraction and X-ray Mössbauer measurement. The ferrimagnetic-to-paramagnetic transition temperature (100 °C) of Mn2FeO4 spinel is different from the tetragonal-to-cubic structure transition temperature (180 °C). The structure transition temperature decreases with increasing pressure. The transition is not coupled with the magnetic transition. Synchrotron X-ray Mössbauer experiments have revealed the pressure effects on the distribution of Fe2+ and Fe3+ at the tetrahedral and octahedral sites in the spinel structure. Ferrimagnetic MnFe2O4 and Mn2FeO4 spinels show sextet spectral features with hyperfine structure elicited by internal magnetic fields. Cubic MnFe2O4 spinel and tetragonal Mn2FeO4 transform to high-pressure orthorhombic postspinel phase above pressures of 18.4 GPa and 14.0 GPa, respectively. The transition pressure decreases with increasing Mn content. The postspinel phase has a paramagnetic property. Mn2O10 dimers of two octahedra are linked via common edge in three dimentional direction. The occupancy of Fe2+ in the tatrahedral site is decreased with increasig pressure, indicating more oredered structure. Consequently, the inverse parameter of the spinel structure is increased with increasing pressure. The magnetic structure refinements clarify the paramagnetic and ferrimagnetic structure of MnFe2O4 and Mn2FeO4 spinel as a function of pressure. The magnetic moment is ordered between A and B sites with the anti-parallel distribution along the b axis. The nuclear tetragonal structure (aN, aN, cN) has the ferrimagnetic structure but the orthorhombic magnetic structure has the ferrimagnetic structure with the lattice constants (aM, bM, cM). The magnetic moment is ordered between A and B sites with the anti-parallel distribution along the bM axis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Important information about plate tectonics and geomagnetic reversals has been derived from measurements of remnant magnetization of spinels in basalts. Spinels are also the most fundamental magnetic compounds in industrial applications. Their magnetic properties, charge transfer and electrical resistivity changing under high-pressure conditions are significant research issues of intensive studies. The iron bearing spinels are the most fundamental magnetic compounds in industrial applications (Fei et al. 1999; Lavina et al. 1994; Jackson et al. 2005; Lin et al. 2013).
Structure analyses of the solid solution between Fe3O4 and Mn3O4 were conducted using thermal neutron diffraction by Hasting et al. (1956); Murasik et al. (1964). They are composed of mixed-charge cations in the tetrahedral (A) and octahedral (B) sites in AB2O4 spinels. Iron-rich members of the Mn3-xFexO4 spinels have a magnetic structure of two-dimensional triangular Yafet-Kittel spin configuration reported by Yafet et al. (1952); Boucher et al. (1971); Gutzmer et al. (1995). Disorder of the cations in two sites has a great influence on magnetic properties such as saturation magnetization, exchange couplings and ferrimagnetic ordering temperatures.
Magnetite undergoes a phase transition to a high-pressure (HP) form, called h-Fe3O4. The stability field and the crystal structure of the HP magnetite, first, show a CaTi2O4-type structure with space group Bbmm (Cmcm) above 25 GPa, and second, defined to be CaMn2O4-type structure (Pbcm). Several experiments have been devoted to magnetite under high-pressure conditions by X-ray powder diffraction (Haavik et al. 2000; Kuriki et al. 2002; Dubrobinsky et al. 2003; Rozenberg et al. 2007; Reichmann et al., 2004). The Verwey transition temperature of Fe3O4 decreases non-linearly with increasing pressure (Todo et al. 2001). A big change in resistivity at the Verwey transition temperature was observed at pressure below 6.5 GPa. The pressure dependence of electrical resistivity of magnetite was measured under 100 GPa (Muramatsu et al. 2016).
The Néel temperatures of Fe3-xMnxO4 solid solutions at ambient pressure are: Fe3O4 (858 K), MnFe2O4 (563 K) and Mn2FeO4 (413 K) Mn3O4 (41.8 K). Mn3-xFexO4 spinel structure transforms to a high-pressure phase of orthorhombic CaMn2O4-type postspinel structure. The transition pressures of the Mn3-xFexO4 solid solution at ambient temperature are: Fe3O4 at 21.8 GPa, MnFe2O4 at 18.4 GPa and Mn3O4 at 10 GPa (Fei et al. 1999; Ye et al. 2015; Yamanaka et al 2022; Paris et al. 1992). The present neutron diffraction and X-ray Mössbauer spectroscopy shed light on the magnetic and structure change under extreme conditions.
Mn3-xFexO4 spinel solid solutions are composed of mixed charged cations of Mn2+, Mn3+, Fe2+ and Fe3+ in the A and B sites in the AB2O4 spinel structure. In the Mn3-xFexO4 solid solution, the spinel phase changes from cubic-to-tetragonal structure with increasing Mn content at ambient pressure. (Van Hook et al., 1958;McMurdie et al. 1950; Wickham 1969, Yamanaka et al. 1973).
Numerous investigations have been conducted on the temperature dependence of the cation distribution in Mn3-xFexO4 spinel solid solution. (Hasting 1956; Rieck et al. 1966). Their phase stabilities and structures under extreme conditions have been studied. (Xu et al. 2004; Kirby et al., 1996; Yamanaka et al. 2001). The Curie temperature of Mn3O4 of about – 250 °C was reported by Boucher et al. (1971); Ole ́s, et al. (1976); Chardon et al. (1986).
Curie temperatures in these spinels increase with increasing Fe content: 140 °C in Mn2FeO4 (synthetic ferrite), 290 °C in MnFe2O4 (jacobsite) and 585 °C in Fe3O4 (magnetite) (Nakagiri et al. 1986; Willerd et al. 1999).
In the present experiment, magnetic and structure studies of MnFe2O4 and Mn2FeO4 were conducted using neutron time-of-flight scattering diffraction under high-pressure condition at PLANET J-PARC (Hattori et al. 2015). Our previous Raman spectroscopic studies and synchrotron X-ray powder diffraction studies of various postspinels have proposed orthorhombic phases of CaFe2O4-type (Pmma), CaTi2O4-type (Cmcm) and CaMn2O4-type (Pbcm) structures as high-pressure polymorphs of different spinels (Yamanaka et al. 2008). Transformations of the oxide spinels are summarized in Table 1. These phases further transform to a new phase by martensitic transformation to a maximal isotropic subgroup structure.
X-ray structure analysis of Mn3-xFexO4 causes an ambiguity, because X-ray atomic scattering factors of Fe (26) and Mn (25) are extremely similar. Neutron diffraction, however, has an effective advantage for the precise diffraction studies of Mn3-xFexO4, because of the big difference in the coherent scattering lengths of Mn (– 3.73 fm) and Fe (9.54 fm). X-ray powder diffraction study at high pressures up to 40 GPa has been also performed by synchrotron radiation at Photon Factory using symmetric diamond anvil pressure cell (DAC) in this experiment.
Mössbauer spectroscopy (MS) study is the effective method to investigate the iron elctronic properties at high pressure. MS allows distingushing between ferric and ferrous ions. High and low spin states of Fe and their relative abundance in substances are clarified. MS studies of high-pressure Fe3O4 (h-Fe3O4) at ambient temperature have been carried out by Pasternak et al. (1994). The cation distributions in spinels at ambient conditions were reported by Mössbauer experiments and NMR studies (Yasuoka et al. 1967; Singh et al. 1981). Hyperfine-structure spectra changes of ferrites were reported as a function of pressure (Kobayashi et al. (2006).
The pressure dependence of the site occupancy and their magnetic structures were determined. In the present study, neutron diffraction at high pressure and high temperature has been conducted. The precise cation distribution has been elucidated by use of the significant difference in coherent scattering lengths between Mn and Fe. Furthermore. Fe2+ and Fe3+ distributions have been clarified by synchrotron X-ray Mössbauer experiments at increasing pressure. We also investigated the electrical resistivity measurement with increasing pressure up to 40 GPa to elucidate the enhancement of electrical conductivity from semiconductor to metal in Mn3-xFexO4 spinel and postspinel with increasing pressure (Yamanaka et al. 2022). The observed enhancement of electrical resistivity with increasing pressure is shown in Supplement file 1.
Experiment
Powder samples of MnFe2O4 and Mn2FeO4 were prepared by solid–solid reaction at ambient pressure. To prepare the samples used for X-ray Mössbauer experiment, isotope-enriched samples with 30% 57Fe content were prepared. The sample preparation is detailed in the supplement file 2.
Neutron diffraction experiment was executed at BL-11 J-PARC (Japan Proton Accelerator Research Complex, Japan Atomic Energy Agency) under high-pressure using spallation neutron time-of-flight (TOF) facility. We used a Paris–Edinburgh (PE) press (VX4) for the experiments at pressures up to 40 GPa at room temperature (Hattori et al. 2019), and also a large-volume six-axis multi-anvil press ATSUHIME at PLANET J-PARC (Sano-Furukawa et al. 2014) for the experiments at pressures to 10 GPa and high temperatures up to 2000 ˚C. Incident neutron wavelength is 0.3 Å–5.8 Å and beam size is 15 mm × 15 mm at maximum.
We performed Rietveld analyses of neutron diffraction data to refine the nuclear structure and magnetic structure. The analysis is conducted using the program GSAS (Larson et al. 1994; Toby 2001). The integrated intensity Io is produced by combination of magnetic scattering factor FM(h) and nuclear scattering factor FN(h):
where s: scale factor, A: absorption, L: Lorentz factor and m: multiplicity.
The nuclear structure factor FN(h) for neutron diffraction is expressed by
where Tj is the temperature factor of j atom. γ indicates the γ-factor of the nuclear magneton.
(γ = 1.913). b is the scattering length ( bMn = – 3.73 fm, bFe = 9.54 fm).
And magnetic scattering factor FM(h) is
where µ is magnetic moment.
The detailed derivation of the neutron diffraction refinement is presented in the Supplement file 3.
Neutron diffraction experiments cannot provide precise information about the distribution of Fe2+ and Fe3+ between the A and B sites in the Mn–ferrite spinel and the M1 and M2 sites of the postspinel structure under high pressure. We measured the synchrotron X-ray Mössbauer spectra of the MnxFe3-xO4 solid solution at SPring-8 BL-10XU (Hirao et al. 2020) under high pressure using micro-beam with the wavelength of 14.4 keV and diamond anvil cell (DAC). A symmetric diamond anvil cell was used to generate high pressure. Ne gas was used as a pressure transmitting media. Rh gasket of 200 µm thick was preindented to 80 µm. High-pressure measurement was performed by ruby-fluorescence scale.
We used the program MossA (Prescher et al. 2012) for the analysis of our Mössbauer spectra and determined the cation distribution as a function of pressure. Deviations from Lorentzian profile shape may have to be fitted using Voigtian (a convolution of Gaussian and Lorentzian functions). The full width half maxim (FWHM) of spectrum is related to the positional disorder of cations.
The isomer shift is referred to the spectrum from zero velocity of the 57Fe spectrum in Fe2O3. The peak positions of the spectra were detected within an error of less than ± 0.05 mm/sec. The internal magnetic fields of the hyperfine structure spectra were determined with the reference of 26.26 T (330 kOe) of the 57Fe in Fe2O3 spectrum.
Result and discussion
Pressure effect on the cooperative Jahn–Teller distortion of Mn2FeO4 spinel phase
Mn3-xFexO4 spinel structure transforms to high-pressure phase of orthorhombic CaMn2O4-type postspinel structure. Present neutron diffraction study reveals the phase diagram of Mn3-xFexO4 under high pressure at ambient temperature, which is presented in Fig. 1. Structure transition from tetragonal to cubic of Mn2FeO4 spinel under pressure at 20 °C is shown as a function of normalized unit-cell volume. Mn2FeO4 and MnFe2O4 spinel transform to postspinel at different pressures at 14.0 GPa and 18.4 GPa, respectively.
Phase diagram of Mn3-xFexO4 under high pressure at ambient temperature is presented by powder neutron diffraction study. Symbol of red stars indicate the X-ray Mössbauer experiment with increasing pressure in the present study at SPring-8. Plotted data with variable chemical compositions at ambient conditions are from Wickham et al. (1969) and Yamanaka ey al. (1973)
Present neutron diffraction patterns of Mn2FeO4 at 2.2 GPa in the heating experiment disclose the tetragonal-to-cubic transition temperature at 180 °C. The cooling experiments show the back transformation from cubic structure to the tetragonal structure at 140 °C. The tetragonal-to-cubic transition is reversible and shows hysteresis, as shown in Fig. 2.
Jahn–Teller effect degradation and appearance in Mn2FeO4 transition between tetragonal and cubic spinel spinels are shown. Neutron diffraction experiment of Mn2FeO4 at 2.2 GPa with increasing temperature confirms the tetragonal-to-cubic transition temperature at 180 °C. In the cooling experiments the cubic structure transforms back to the tetragonal structure at 140 °C. The tetragonal-to-cubic transition is reversible and shows a hysteresis of the transition
Mn3+ (3d4) prefers octahedral configuration in the strong ligand field. In the Mn3-xFexO4 solid solution, Mn-rich phases present lattice distortion due to the cooperative Jahn–Teller (JT) effects. At ambient pressure, Mn3O4 transforms from tetragonal (I41/amd z = 4) to cubic (Fd_3m z = 8) at 1170 °C (McMurdie et al. 1950) with vanishing JT effect. The phase has an elongated structure along the c-axis with c/a > 1.
The tetragonal-to-cubic transition temperature of Mn2FeO4 is 160 °C at 3 GPa and 180 ℃ at1GPa by the temperature dependence of c/a. These experiments prove the transition temperature decreases with increasing pressure. P–T boundary between the tetragonal and cubic phases has a negative slope. The distortion of the elongation along the c-axis disappears in the cubic Mn2FeO4. The lattice distortion may be reduced with increasing temperature and finally the lattice constant ratio becomes c/a = 1. resulting in the transformation to the cubic symmetry (c = a).
The two-phase mixtures with postspinel are found in the transition region (Fig. 3). The normalized unit-cell volumes of the three phases of cubic, tetragonal spinel and orthorhombic postspinel are shown in the figure. Both spinels have two-phase mixture regions with high-pressure postspinel phase.
The present transition case from tetragonal-to-cubic phase of Mn2FeO4 is extremely rare for the JT transition under compression. The following spinels: Fe2TiO4 (Yamanaka et al. 2013), FeCr2O4 (Kyono et al. 2011a, b), ZnMn2O4 (Choi et al. 2006), CuMn2O4 (Waskowska et al. 2001), CuFe2O4 (Kyono et al., 2015), ZnGa2O4 (Errandonea et al. 2009), NiMn2O4 (Åsbrink et al., 1988), transitions from cubic-to-tetragonal spinel show the with increasing pressure. Many of them have the tetragonal distortion with flattened octahedral distortion along the c-axis (c/a < 1). The octahedral site of the tetragonal phase of Mn2FeO4 is elongated along the c-axis and the lattice constant ratio c/a > 1 according to the crystal field stabilization energy (CFSE). Topological presentation is developed from the JT distortion. Pressure dependence of cooperative JT distortion in Mn2FeO4 is caused by localized orbital electronic states of Mn3+ under extreme conditions.
The result of the structure refinements of cubic MnFe2O4 and tetragonal Mn2FeO4 are presented in Supplement Table 1 and Supplement Table 2. The site occupancies are also presented in these tables. Their bond distances of A–O and B–O together with AO4 tetrahedral volume and BO6 octahedral volume are presented in the tables.
The magnetic interaction is induced by supper exchange mechanism via oxygen. A–O and B–O bond distances and A–O–B bond angle are strongly related for the mechanism.
The compressions of the inter-nucleus distances A–A, A–B and B–B are strongly affected to the supper exchange. The B–B distance is much smaller than the other two A–A and A–B distances (Fig. 4). Then, the super-exchange between the B and the adjacent B cation is easier to gain extremely low resistivity. With compression, B–B shorter distance promotes a higher conduction. The B–B super-exchange model in the corner sharing octahedra is shown in Fig. 5. Super exchange for the electron hop** between Fe2+ and Fe3+ ion is more possible.
Super exchange for the electron hop** between Fe2+ and Fe3+ ion is more possible in the B site than the A site. The super-exchange between B and the adjacent B cations is easier to gain extremely low resistivity. With compression, B–B shorter distance promotes a higher conduction. The B–B super-exchange model is shown in the corner sharing octahedra
Cation ordering
MnFe2O4 and Mn2FeO4 compounds are composed of the mixed-charge elements and their ionic radii are similar. According to the effective ionic radii (Shannon et al. 1969) and cation site preference (Duniz et al. 1957, 1960), positional change of these cations may be possible at high pressure. The distortions of the tetrahedral and octahedral sites make cation exchange possible by compression.
The cation exchange is common at high temperature and thermal atomic vibration is a strong mechanism for the cation positional change. Charge transfer in Fe2+, Fe3+, Mn2+ and Mn3+ is often observed in many experiments. Neutron diffraction studies show the change of the site occupancies at the A and B site and A–O and B–O distances at extreme high pressures. The bond distances at the A and B sites of Mn–O and Fe–O were evaluated through neutron diffraction study.
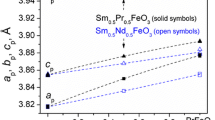
The site occupancies have a significant effect on electrical conductivity. The magnetic moments of the A and B sites in the MnFe2O4 and Mn2FeO4 spinel structures were also clearly observed. The inverse parameter i of the spinel structure in (Mn2+iFe3+1-i)[Mn3+1-iFe2+1-iFe3+2i]O4 could be precisely defined for MnFe2O4. At high pressure, just before the transition pressures of the respective samples, they more closely adopt the normal spinel structure with i = 1. The inverse parameter of MnFe2O4 is presented as a function of pressure in Fig. 6.
Mössbauer resonance experiment also shows Fe2+ and Fe3+ positional change in the A and B site at high pressure. Mössbauer spectra indicate two hyperfine structures of sextet patterns indicating ferrimagnetic moment of the A and B sites of MnFe2O4 cubic spinel at 0.25 GPa and 300 K in Fig. 7. The spectrum of the A site is assigned to Fe3+ in the tetrahedral site. The spectrum of the octahedral site (B) site is assigned to mixture of Fe3+ and Fe2+. These Fe3+ and Fe2+ cations in the B site are not individually separated because of the electron hop** between these cations.
Mössbauer spectrum of MnFe2O4 at 0.25GPa. Mössbauer spectrum of MnFe2O4 cubic spinel at 0.25 GPa and 300 K is shown. The spectra indicate two hyperfine structures of sextet patterns indicating ferrimagnetic moment of the A and B sites. The spectrum of the A site is assigned to Fe3+ in the tetrahedral site. The spectrum of the B site is assigned to mixture of Fe3+ and Fe2+ in the octahedral site. Intensity spectra ratio of the A and B site is presented
Mössbauer spectra of MnFe2O4 cubic spinel at 10.0 GPa and 300 K are shown in Fig. 8 and they indicate two hyperfine structures of sextet patterns indicating ferrimagnetic moment of the A and B sites, which is a very similar spectra of the spectra in Fig. 7. Internal magnetic field observed from the both sextets at A and B sites are not changed between two pressures, 0.25 and 10.0 GPa.
Mössbauer spectrum of MnFe2O4 at 10.0 GPa and 300 K. Mössbauer spectrum of MnFe2O4 cubic spinel at 12.5 GPa and 300 K. The spectra show very similar hyperfine structures of sextet patterns to the spectra at 0.25 GPa shown in Fig. 5
Under further compression at 17.0 GPa 300 K shown in Fig. 9, Mössbauer spectrum of MnFe2O4 cubic ferrimagnetic phase shows one doublet indicates the Fe3+ spectrum at the A site and one sextet shows the Fe2+ and Fe3+ spectrum at the B site. The former spectrum proves neither hyperfine structure and nor internal magnetic field. The spectrum of the hyperfine structure at the A site is not observed but the spectrum at the B site shows the internal magnetic field.
Mössbauer spectrum of MnFe2O4 cubic ferrimagnetic phase at 17.0 GPa 300 K. One doublet indicates the Fe3+ spectrum at the tetrahedral site One sextet shows the Fe2+ and Fe3+ spectrum at the octahedral site are shown. The former spectrum proves neither hyperfine structure and nor internal magnetic field
Neutron diffraction study of MnFe2O4 and Mn2FeO4 spinels indicates the ferrimagnetic cubic spinel structure at pressures up to 17.4 GPa and 14.0 GPa, respectively. Mössbauer spectra do not show any remarkable change up to 12.5 GPa. However, at 17.0 GPa the spectrum changes from sextet to doublet, proving neither hyperfine structure and nor internal magnetic field and magnetic moment of magnetic ions disappears, as shown in Fig. 9. Peak width of the sextet spectrum becomes broad at 17.0 GPa. The positional disorder of magnetic ions in the octahedral (B) site induces the peak broadening of the large FWHM in the spectrum.
Mössbauer spectra of the orthorhombic postspinel Mn2FeO4 at 18.0 GPa and 300 K have two doublets, as shown in Fig. 10. One indicates the sixfold octahedron indicating Fe3+ in the octahedral site (M2 site). Another doublet represents Fe2+ in the highly distorted eightfold large cation site (M1 site) with good reason of a large quadruple splitting. Two doublets of the Mössbauer spectra of postspinel indicate paramagnetic behavior. The result of Fe2+ and Fe3+ distributions in the VIIIM1 and VIM2 sites is expressed by the following cation distribution: VIII[Mn2+0.746,Fe2+0.256]VI(Mn3+0.628,Fe3+0.372)2O4. There is no sextet spectra in the postspinel phases of Mn2FeO4 and MnFe2O4 proving they are paramagnetic at high pressures.
Mössbauer spectrum of orthorhombic postspinel Mn2FeO4 spectra at 18.0 GPa. Two doublets are presented: Inner larger doublet indicates the sixfold octahedron, which is assigned to Fe3+ in the M2 site. Outer doublet represents Fe2+ in the highly distorted eightfold large cation site (M1 site) and postspinel is paramagnetic
In the Mössbauer spectra, the relative intensities of individual components possibly give a suggestion of the corresponding population. The intensities of the various peaks reflect the relative concentrations of cations. Observed site occupancies are estimated from the peak intensities of the Mössbauer spectra of MnFe2O4 and Mn2FeO4 at various pressures. The following data: (1) the isomer shift (δ), (2) the quadruple splitting (Δ) and (3) the magnetic hyperfine field (Bhf) are presented in Table 2. Peak intensity (Int) ratios of two or three spectra are also presented.
Two sextets of the spectra of MnFe2O4 cubic spinel at pressures from 1 atm to 12.5 GPa do not show a big difference in their peak intensities in Table 2 but show a slight increase in the intensity ratio with increasing pressure. The hypothetical structure proposed from the intensity ratio of two sextet spectra of the MS experiment gives the cation distribution, which is somewhat different from the result of the neutron diffraction structure analysis.
The distortion of Mn2FeO4 and MnFe2O4 spinels under compression are related to their elastic properties. The compression behavior of Mn3-xFexO4 spinels can be described by third-order Birch–Murnaghan equation of state. The equation of state is given by
where P is the pressure, V0 is the reference volume, V is the deformed volume with respect to pressure,, B0 is the bulk modulus, and B0' is the derivative of the bulk modulus. The bulk modulus and its derivative are usually obtained from fits to experimental data and are defined as
The equations of state of MnFe2O4 and Mn2FeO4 show the difference in their transition pressures. Bulk modules of Mn3-xFexO4 spinel solid solutions are presented in Table 3.
MnFe2O4 and Mn2FeO4 are characterized by cation disorder and are more compressible than the end-members of the solid solution. MnFe2O4 is less compressible than Mn2FeO4.
High-pressure polymorph postspinel phase
Magnetite undergoes a phase transition to a high-pressure (HP) form, called h-Fe3O4 above 25 GPa and defined to be postspinel of CaMn2O4 (Pbcm) structure. Postspinel is characterized by herringbone structure, shown in Fig. 11. The present X-ray Mössbauer spectra analyses and neutron diffraction experiments describe the cation distributions of Mn and Fe. The structure refinements of postspinel of Mn2FeO4 at pressures up to 26.8 GPa are presented in Supplement Table 3. (The refinement of the quenched sample is also presented in the table.) The VIIIM1 site volume is much larger than the VIM2 site. The VIIIM1 site of Mn2FeO4 and MnFe2O4 postspinel is mostly occupied by Mn2+, which increases with increasing pressure. The VIM2 site of MnFe2O4 is occupied by mainly Fe and only small amount of Mn. On the other hand, the VIM2 of Mn2FeO4 is half-occupied by Mn. The phase of Mn2FeO4 is composed of an almost ideal ordered structure of VIII[Mn2+]VI(Mn3+0.5Fe3+0.5)2O4. The atoms in the VIM2 site are located on the edge-sharing plane perpendicular to the c-axis, as shown in Fig. 11. Mn2O10 dimers of two octahedra in the structures are linked via common edges.
Magnetic structure of Mn2FeO4 and MnFe2O4 spinel
Present magnetic structure analysis of Mn2FeO4 and MnFe2O4 as a function of pressure is executed using the neutron diffraction intensity Io. Integrated intensity Io is a combination of both magnetic scattering factor |FM(h) |2 and nuclear scattering factor |FN(h)|2 by Eq. (1). Present Rietveld refinement based on the ferrimagnetic structure discloses the tetrahedral and octahedral symmetry of the tetragonal-to-cubic transition with increasing pressure. The site symmetry 0.2 m. of the octahedral (B) site in the tetragonal symmetry of I41/amd changes to the symmetry of._3m of the cubic symmetry of Fd_3m.
The diffraction peaks of 101 and 112 of tetragonal phase have a large contribution of magnetic scattering. The temperature evolution of the diffraction intensity of Mn2FeO4 at ambient pressure indicates that both peak intensities suddenly drop around 100 °C, which is displayed in Fig. 12. The magnetic transition temperature at 100 °C of ferrimagnetic-to-paramagnetic is different from the tetragonal-to-cubic structure transition temperature at 180 °C. The magnetic transition temperature is lower than that of the nuclear structure transition. The structure change is not coupled with magnetic transition.
The present magnetic refinement shown in Table 4 confirms the site occupancies at the A and B sites of Mn2FeO4 postspinel under elevating pressure. Magnetic moment distribution of the spinel and postspinel structure requires the effective magnetic susceptibility of cations. Effective Bohr magneton is used from extant published data defined by previous experiments: Mn2+ (5.92 µB), Mn3+ (4.90 µB), Fe2+ (4.90 µB) and Fe3+ (5.92 µB) (Neel 1948). Iron-rich members of the Mn3-xFexO4 spinels have the magnetic structure of two-dimensional Yafet-Kittel triangular spin configuration. The magnetic structure of a powder sample of MnFe2O4 was determined by thermal neutron diffraction (Levy 2015). Spin moments of the A and B sites of the iron substitution on the magnetic property were clarified at temperatures 10 K and 295 K (Baron et al. 1998).
The influence of iron substitution on the magnetic properties is clarified in both ordered and disordered ferrimagnetic spinel-phases. Magnetic cations of Mn and Fe are located at the crystallographically special position in the tetragonal and cubic structure. Positional parameters of these cations are variable and magnetic moments are also variable parameters besides thermal variables. The reliability factor for the refinement is enhanced by the inclusion of magnetic moment effect. Nuclear structure analysis without consideration of magnetic moment effect was not converged properly.
Several possible magnetic space groups in nine subgroups of the tetragonal spinel structure of I41/amd are examined for the observed intensities. The magnetic space group of I41/am’d’ is the most reliable subgroup of the space group symmetry of I41/amd. Rietveld refinement of Mn2FeO4 at 2.2 GPa 20 ℃ tetragonal I41/amd with the paramagnetic structure model is χ2 = 10.51 and R(F2) = 0.211. We analyzed the diffraction intensities of the observed data in this study, because the quality of the data taken at high pressures in this study is not sufficient to discuss the detail of counted spins. The ferrimagnetic structure model based on the orthorhombic space group Pmma, (which is of an isomorphic subgroup of I41/amd) shows much better agreement of χ2 = 2.157 and R(F2) = 0.117. The lattice constants (aM, bM, cM) of Mn2FeO4 of the present orthorhombic ferrimagnetic structure are derived from the nuclear tetragonal structure (aN, aN, cN). The bM cell edge is twice larger than bN of the paramagnetic lattice, because the magnetic moment is ordered between A and B sites with the anti-parallel distribution along the bM axis. The Rietveld analysis is shown in Fig. 13. The distribution of the anti-parallel magnetic spins in A and B site are presented in the figure. Pressure dependence of site occupancy and magnetic moment of the ferrimagnetic Mn2FeO4 spinel are summarized in Table 4. The site occupancies of Mn at the tetrahedral and octahedral sites of Mn2FeO4 and MnFe2O4 spinels are shown with increasing pressure. The cation distribution becomes close to the ordered normal spinel structure. Ferrimagnetic spin moment becomes smaller at higher pressure before the transformation to the high-pressure postspinel phase.
Paramagnetic Mn2FeO4 tetragonal structure at 2.2 GPa is shown in the left figure. Right figure shows anti-parallel magnetic spin moments along the cM axis of the ferrimagnetic Mn2FeO4 orthorhombic structure at 2.2 χ2R(F2) = 0. 117). Only cations are presented in the figure. They are similar positions in the paramagnetic tetragonal structure. Ferrimagnetic lattice has aM, bM, cM., and bM is twice large bN of the paramagnetic lattice
Pressure dependence of magnetic structure was suggested by X-ray Mössbauer experiment. Deviations from ian profile may be induced from variations of local environments or fluctuations of parameters.
Conclusion
Magnetic studies of minerals are reliable witnesses of paleomagnetism by high-resolution studies of these structures. The magnetic structures are built during the cooling of molten rock and reflect the earth's magnetic field at the time of their formation. This record provides information on the past behavior of Earth's magnetic field and geomagnetic reversal. The magnetic properties are reset by the interaction of the magnetic spin inside the Earth's magnetic field. The geomagnetic reversal is an indicator of magnetic field change in plate tectonics. However, the pressure effect of the plate tectonics still remains to be seen in the magnetic study of minerals.
The present neutron diffraction and synchrotron X-ray Mössbauer spectroscopic study provide the comprehension of the magnetic and structure change under extreme conditions. Some of oxide spinels with transition elements have a ferrimagnetic property at ambient conditions. They transform to the postspinel structures under high-pressure condition. Cation distributions in Mn3-xFexO4 solid solutions under extreme conditions are significant research subjects not only for geophysical understands such plate tectonics and geomagnetic reversals but also for the basic magnetic ferrite industrial materials. Magnetic and structure transition studies are possible by neutron time-of-flight scattering diffraction at PLANET J-PARC. Besides the neutron diffraction, X-ray Mössbauer experiment is a significant and complementary study to investigate the magnetic property of Mn3-xFexO4 solid solutions from the hyperfine structure of Zeeman splitting. The present experiments disclosed the following new discoveries of magnetic properties:
(1) The lattice distortion is not coupled with magnetic transition. The magnetic transition temperature from ferrimagnetic-to-paramagnetic of spinels is lower than the structure transition temperature from tetragonal-to-cubic structure transition. The structure transition temperature decreases with increasing pressure.
(2) Pressure dependence of cooperative Jahn–Teller distortion in Mn2FeO4 is observed by the interaction between localized orbital electronic states of Mn3+ and the compression of the octahedral site. The transition temperature from tetragonal-to-cubic phase decreases with increasing pressure. The transition of Mn2FeO4 is an extremely rare case for the JT transition with increasing pressure. Generally many spinels show cubic-to-tetragonal transition at high pressure and the tetragonal distortion is of flattering octahedral distortion of c/a < 1, However, the tetragonal phase of Mn2FeO4 shows the transformation from tetragonal-to-cubic and the octahedral site is elongated along to the c-axis and the lattice constant ratio is c/a > 1.
(3) Inverse parameter change under compression.
X-ray Mössbauer measurement and nuetron diffraction study confirm that the occupancy of Fe2+ in the tatrahedral site is decreased with increasig pressure, indicating more oredered structure. The inverse parameter is increased with increasing pressure.
(4) The cubic MnFe2O4 spinel and tetragonal Mn2FeO4 transform to the high-pressure orthorhombic postspinel phase at pressure 18.4 GPa and 14.0 GPa, respectively. The transition pressure decreases with increasing Mn content. The observed charge distribution of postspinel becomes an almost ideally ordered structure expressed by VIII[Mn2+]VI(Mn3+0.5Fe3+0.5)2O4, transformed from cubic Mn2FeO4 spinel.
(5) The magnetic refinements clarify the paramagnetic and ferrimagnetic structure of MnFe2O4 and Mn2FeO4 spinel as a function of pressure. The magnetic moment is ordered between A and B sites with the anti-parallel distribution along the b axis.
References
Akaogi M, Hamada Y, Suzuki T, Kobayashi M, Okada M (1999) High pressure transitions in the system Mg2AlO4 –CaAl2O4 a new hexagonal aluminous phase with implication for the lower mantle. Phys Earth Planet Inter 115:67–77
Akaogi M, Tajima T, Okano M, Kojitani H (2019) High-Pressure and High-temperature phase transitions in Fe2TiO4 and Mg2TiO4 with Implications for titanomagnetite inclusions in superdeep diamonds. Minerals 9(10):614
Andrault D, Casanova NB (2001) High-pressure phase transformations in the MgFe2O4 and Fe2O3-MgSiO3 systems. Phys Chem Min 28:211–217
Åsbrink S, Waskowaka A, Olsen JS, Gerward L (1998) High-pressure phase of the cubic spinel NiMn2O4. Phys Rev B 57:4972–4974
Baron V, Gutzmer J, Rundlof H, Tellgren R (1998) The influence of iron substitution on the magnetic properties of hausmannite, Mn2+(Fe, Mn)3+2 O4. Am Min 83:786–793
Boucher B, Buhl R, Perrin M (1971) Magnetic structure of Mn3O4 by neutron diffraction. J Applied Physics 42:1615–1617
Chardon B, Vigneron F (1986) Mn3O4 commensurate and incommensurate magnetic structures. J Magn Magn Mater 58:128–134
Choi H, Shim JH, Min BI (2006) Electronic structures and magnetic properties of spinel ZnMn2O4 under high pressure. Phys Rev B 74:172103
Darul J, Lathe C, Piszora P (2013) Mn3O4 under high pressure and temperature: thermal stability, polymorphism, and elastic properties. J Phys Chem C 117:23487–23494
Dubrobinsky LS, Dubrovinskaia NA, McCammon R, Kh G, Ahuja R, Osorio-Guillen JM, Dmitriev V, Weber DP, Bihan TL, Johansson B (2003) The structure of the metallic high pressure Fe3O4 polymorph experimental and theoretical study. J Phys: Condens Matter 16:7697
Dunitz JD, Orgel LE (1957) Electronic properties of transition-metal oxides I. Distortion from cubic symmetry. J Phys Chem Solids 3:20–29
Dunitz JD, Orgel LE (1960) Stereochemistry of ionic solids. Adv Inorg Chemis Radiochem 2:1–60
Errandonea D, Kumar RS, Manjon FJ, Ursaki VV, Rusa EV (2009) Post-spinel transformations and equation of state in ZnGa2O4: Determination at high-pressure by in situ x-ray diffraction. Phys Rev B 79:024103
Fei Y, Mao HK, Hemley RJ, Shu J, Shen G (1999) In situ structure determination of the high-pressure phase of Fe3O4. Am Miner 84:203–206
Gutzmer J, Beukes NJ, Kleyenstuberanda AS, Burger E (1995) Magnetic hausmannite from hydrothermally altered manganese ore in the paleoproterozoic kalahari manganese deposit, Transvaal super group, South Africa. Mineral Magazin 59:703–716
Haavik C, Sttoen S, Fjelvag H, Hanfland M, Häuserman D (2000) Equation of state of magnetite and its high-pressure modification: Thermodynamics of the Fe-O system at high pressure. Am Miner 85:514–523
Hasting JM, Corliss LM (1956) Neutron diffraction study of manganese ferrite. Phys Rev 104:328–331
Hattori T, Sano-Furukawa A, Arima H, Komatsu K, Yamada A, Inamura Y, Nakatani T, Seto Y, Nagai T, Utsumi W, Iitaka T, Kagi H, Katayama Y, Inoue T, Otomo T, Suzuya K, Kamiyama T, Arai M, Yagi T (2015) Design and Performance of High-Pressure PLANET Beamline at Pulsed Neutron Source at J-PARC. Nucl Instrum Methods Phys Res Sec A 780:55–67
Hattori T, Sano-Furukawa A, Machida S, Abe J, Funakoshi K, Arima H, Okazaki N (2019) Development of a technique for high-pressure neutron diffraction at 40 GPa with a Paris-Edinburgh press. High Press Res 39:417–425
Hirao N, Kawaguchi SI, Hirose K, Shimizu K, Ohtani E, Ohishi Y (2020) New developments in high-pressure X-ray diffraction beamline for diamond anvil cell at SPring-8. Matter Radiat Extremes 5:018403
Ishii T, Kojitani H, Tsukamoto S, Fu**o K, Mori D, Inaguma Y, Tsu**o N, Yoshino T, Yamazaki D, Higo Y, Funakoshi K, Akaogi M (2014) High-pressure phase transitions in FeCr2O4 and structure analysis of new postspinel FeCr2O4 and Fe2Cr2O5 phases with meteoritic and petrological implications. Am Miner 99:1788–1797
Jackson JM, Strhahn W, Shen G, Zhao J, Hu MY, Errandonea D, Bass JD, Fei Y (2005) A synchrotron Mössbauer spectroscopy study of (MgFe)SiO3 perovskite up to 120 GPa. Am Miner 90:199–205
Kirby SH, Stein S, Okai EA, Rubie DC (1996) Metastable mantle phase transformations and deep earthquakes in subducting oceanic lithosphere. Review of Geophysics 34:261–306
Kuriki A, Moritomo Y, Ohishi Y, Kato K, Nishibori E, Takata M, Sakata M, Hamada N, Todo S, Mori N, Shimomura O, Nakamura A (2002) High-pressure structure analysis of Fe3O4. J Phys Soc Japan 71:3092–3093
Kobayashi H, Isogai K, T., Hamada, N., Onodera, H. and Todo, S. (2006) Structure properties of magnetite under high pressure studied by Mössbauer spectroscopy. Phys Rev B 73(10):104110
Kyono A, Ahart M, Yamanaka T, Gramsch S, S., Mao, H.k. and Hemley, R.J. (2011a) High-pressure Raman spectroscopic studies of ulvöspinel Fe2TiO4. Am Mineral 96:1193–1198
Kyono A, Gramsch SA, Yamanaka T, Ikuta D, Ahart M, Mysen BO, Mao HK, Hemley RJ (2011b) The influence of the Jahn-Teller effect at Fe2+ on the structure of chromite at high pressure. Phys Chem Miner. https://doi.org/10.1007/s00269-011-0468-6
Larson AC, Von Dreele RB (2004) General Structure Analysis System (GSAS), Los Alamos National. Laboratory Report LAUR 86-748
Lavina B, Salviulo G, Giusta AD (1994) Cation distribution and structure modeling of spinel solid solutions. Phys Chem Miner 29:10–18
Levy D, Pastern L, Holster A, Visvovo G (2015) Thermal expansion and cation partitioning of MnFe2O4 (Jacobsite) from1.6 to1276K studied by using neutron powder diffraction. Solid State Chem 201:15–19
Lin JF, Speziale S, Mao Z, Marquardt H (2013) Effects of the electronic spin transitions of iron in lower-mantle minerals; implications to deep-mantle geophysics and geochemistry. Rev Geophys 51:244–275
Malavasi L, Tealdi C, Amboage M, Mozzatti MC, Flor G (2005) High pressure X-ray diffraction study of MgMn2O4 tetragonal spinel. Nuclear instrument and methods. Phys Res B 238:171–174
McMurdie HF, Sullvan BM, Maur FA (1950) High temperature X-ray study of the system Fe3O4–Mn3O4. J Res NIST 45:35–41
Muramatsu T, Gasparov LV, Berger H, Hemley RJ, Struzhkin VV (2016) Electrical resistance of single-crystal magnetite (Fe3O4) under quasi-hydrostatic pressures up to 100 GPa. J Appl Phys 119:135903
Murasik A, Roult G (1964) Structure magnetique du manganite de fer par diffraction neutronique. J De Phzs 25:522–525
Nakagiri N, Manghnani MH, Ming LC, Kimura S (1986) Crystal structure of magnetite under pressure. Phys Chem Minerals 13:238–244
Néel L (1948) Propriétés magnétiques des ferrites; ferrimagnétisme et antiferromagnétisme. Ann Phys (parris) 3:137
Ole ́s, A., Kajzar, F., Kucab, M. and Sikora, W. (1976) Magnetic Structures. Panstwowe Wydawnictwo Naukowe, Determined by Neutron Diffraction
Paris E, Ross CR, Olijnyk H (1992) Mn3O4 at high pressure: a diamond-anvil cell study and a structural modeling. Eur J Mineral 4:87–93
Pasternak MP, Nasu S, Wada K, Endo S (1994) High-pressure phase of magnetite. Phys Rev B 50:6446
Prescher C, McCammon C, Dubrovinsky L (2012) MossA: a program for analyzing energy-domain Mössbauer spectra from conventional and synchrotron sources. J App Crystallogr 45:329–331
Reichmann HJ, Jacobsen SD (2004) High-pressure elasticity of a natural magneite crystal. Am Miner 89:1061–1066
Ricolleau A, Fei Y (2016) Equation of state of the high-pressure Fe3O4 phase and a new structural transition at 70 GPa. Am Miner 101:719–725
Rieck GD, Driessens FCM (1966) The structure of manganese-tron-oxygen spinels Acta. Crystallography 20:521–525
Rozenberg GK, Amiel Y, Xu WM, Pasternak MP, Jeanloz R, Hanfland M, Taylor RD (2007) Structure characterization of temperature-pressure-induced inverse normal spinel transformation in magnetite. Phys Rev B 75:020102
Sano-Furukawa A, Arima H, Yamada A, Tabata S, Kondo M, Nakamura A, Kagi H, Yagi T (2014) Six-axis multi-anvil press for high-pressure, high-temperature neutron diffraction experiments. Rev Sci Instrum 85:113905
Shannon RD, Prewitt CT (1969) Effective ionic radii in oxide and Fluorides. Acta Crystallogr B 25:935
Singh VK, Khatri NK, Lokanathan S (1981) Mössbauer study of ferrite systems CoxMn1-xFe2O4 and NixMn1-xFe2O4. Solid State Phys 16:273–280
Toby BH (2001) EXPGUI, a graphical user interface for GSAS. J Appl Crystallogr 34:210–213
Todo S, Takeshita N, Kanehara T, Mori T, Mori N (2001) Metallization of magnetite (Fe3O4) under high pressure. J Appl Phys 89:7347
Van Hook HJ, Keith ML (1958) The system Fe3O4-Mn3O4. Am Miner 43:69–83
Wang ZO, H, S.C., Lazor, P. and Saxena, S.K. (2002) High pressure Raman spectroscopic study of spinel MgCr2O4. J Phys Chemis Solid 63:2057–2061
Wang Z, Downs RT, Pischedda V, Shetty R, Saxena SK, Zha CS, Zhao YS, Schiferl D (2003a) High-pressure x-ray diffraction and Raman spectroscopic studies of the tetragonal spinel CoFe2O4. Phys Rev B 66:024103
Wang Z, Schiferl D, Zhao Y, O’Neil H, St C (2003b) High pressure Raman spectroscopy of spinel-type ferrite ZnFe2O4. J Phys Chemis Solids 64:2517–2523
Waskowska A, Gerward L, Olsen JS, Steenstrup S, Talik E (2001) CuMn2O4: properties and the high-pressure induced Jahn-Teller phase transition. J Phys Condens Matter 13:2549–2562
Wickham DG (1969) The chemical composition of spinels in the system Fe3O4-Mn3O4. J Inorg Nucl Chem 31:313–320
Willerd M, Nakamura Y, Laughling DE, McHenry ME (1999) Magnetic properties of ordered and disordered spinel-phase ferrimagnets. J Am Ceram Soc 82:3342–3346
Wu Y, Wu X, Qin S (2012) Pressure-induced phase transition of Fe2TiO4: X-ray diffraction and Mössbauer spectroscopy. J Solid State Chem 185:72–75
Xu Y, Poe BT, Shankland J, Rubie DC (1998) Electrical conductivity of olivine, wadsleyite, and ringwoodite under upper mantle conditions. Science 280:1415–1418
Xu WM, Machavariani GYu, Rozenberg GKh, Paternak MP (2004) Mössbauer and resistivity studies of the magnetite and electronic properties of the high-pressure phase of Fe3O4. Phys Rev B 70:174106
Yafet Y, Kittel C (1952) Antiferromagnetic Arrangements in Ferrites. Phys Rev 87:290–294
Yamanaka T, Nakahira M (1973) Dependence of the cation distribution in manganese ferrite, MnFe2O4, on temperature and oxidation. Mineral J 73:202–220
Yamanaka T, Okita M (2001) Magnetic properties of the Fe2SiO4-Fe3 O4 spinel solid solution. Phys Chem Min 28:102–109
Yamanaka T, Uchida A, Nakamoto Y (2008) Structural transition of postspinel phases CaMn2O4, CaFe2O4 and CaTi2O4 under high pressures up to 80 GPa. Am Miner 93:1874–1881
Yamanaka T, Kyono A, Nakamoto Y, Meng Y, Kharlamova S, Struzhkin VV, Mao HK (2013) High-pressure phase transitions of Fe3-xTixO4 solid solution up to 60 GPa correlated with electronic spin transition. Am Miner 98:736–744
Yamanaka T, Rahman S, Nakamoto Y, Hattori T, Jang BG, Kim DY, Mao HK (2022) Enhancement of electrical conductivity to metallization of Mn3-xFexO4 spinel and postspinel with elevating pressure. Phys Chem Solid. https://doi.org/10.1016/j.jpcs.2022.110721
Yasuoka H, Hirai H, Shinjo T, Kiyama M, Bando Y, Takada T (1967) NMR determination of metal ion distribution in manganese ferrite prepared from aqueous solution. J Phys Soc Jpn 22:174–180
Ye L, Zhai S, Wu X, Xu C, Yang K, Higo Y (2015) Compressibility of MnFe2O4 polymorphs. Phys Chem Miner 42:569–577
Acknowledgements
We would like to express our great thanks to Prof. Yukio Noda of Institute of Multidisciplinary Research for Advanced materials Tohoku University Japan and to Dr. Yoshihisa Ishikawa of Institute of Materials Structure Science KEK Japan for their fruitful discussion about magnetic structure. The present investigation was performed under the auspice of J-PARC Proposal No 2016B0018.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yamanaka, T., Hirao, N., Nakamoto, Y. et al. Magnetic and structure transition of Mn3-xFexO4 solid solutions under high-pressure and high-temperature conditions. Phys Chem Minerals 49, 41 (2022). https://doi.org/10.1007/s00269-022-01215-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00269-022-01215-4