Abstract
Interleukin (IL)-33 is an important cytokine in the tumour microenvironment; it is known to promote the growth and metastasis of solid cancers, such as gastric, colorectal, ovarian and breast cancer. Our group demonstrated that the IL-33/ST2 pathway enhances the development of squamous cell carcinoma (SCC). Conversely, other researchers have reported that IL-33 inhibits tumour progression. In addition, the crosstalk between IL-33, cancer cells and immune cells in SCC remains unknown. The aim of this study was to investigate the effect of IL-33 on the biology of head and neck SCC lines and to evaluate the impact of IL-33 neutralisation on the T cell response in a preclinical model of SCC. First, we identified epithelial and peritumoural cells as a major local source of IL-33 in human SCC samples. Next, in vitro experiments demonstrated that the addition of IL-33 significantly increased the proliferative index, motility and invasiveness of SCC-25 cells, and downregulated MYC gene expression in SCC cell lines. Finally, IL-33 blockade significantly delayed SCC growth and led to a marked decrease in the severity of skin lesions. Importantly, anti-IL-33 monoclonal antibody therapy increase the percentage of CD4+IFNγ+ T cells and decreased CD4+ and CD8+ T cells secreting IL-4 in tumour-draining lymph nodes. Together, these data suggest that the IL-33/ST2 pathway may be involved in the crosstalk between the tumour and immune cells by modulating the phenotype of head and neck SCC and T cell activity. IL-33 neutralisation may offer a novel therapeutic strategy for SCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Interleukin (IL)-33 is a pleiotropic cytokine that modulates the activities of tumour and immune cells [1]. IL-33 signals through the ST2 receptor, which exists in two forms, a soluble form (sST2), which acts as a decoy receptor, sequesters free IL-33 and does not signal, and a membrane bound form (ST2), which activates the MyD88/NF-κB signalling pathway to modulate immune cell functions [2].
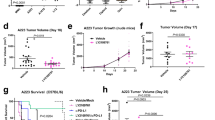
Within the tumour microenvironment, epithelial cells, dendritic cells, macrophages, myeloid-derived suppressor cells (MDSCs), fibroblasts and cancer cells produce IL-33 [Animal model of SCC The Animal Care and Use Committee of the Bauru School of Dentistry, University of São Paulo, approved the animal procedures [012/2017]. All animals were maintained in compliance with the Guide for the Care and Use of Laboratory Animals prepared by CONCEA. Eight-week-old female BALB/c mice were obtained from the School of Medicine of São Paulo of Ribeirão Preto, University of São Paulo. The mice were subjected to a two-stage carcinogenesis procedure [14]: 75 µg of 7,12-dimethylbenz[a]anthracene (DMBA; Sigma-Aldrich, St. Louis, MO, USA) was applied topically to the shaved back skin of wild-type BALB/c mice (n = 18), followed by twice-weekly topical application of 10 µg of the tumour promoter phorbol 12-myristate 13-acetate (PMA, Sigma-Aldrich). Control IgG and anti-IL-33 antibody (AF3626) were obtained from R&D Systems. For the in vivo experiments, the control antibody or the anti-IL-33 antibody were administered intraperitoneally once per week at a dose of 3 µg/mice starting 16 weeks after DMBA exposure [15]. The mice were euthanised 17 weeks after the first PMA cycle, and tumours were harvested for analysis. After euthanising the mice, the skin and the tumour-draining lymph nodes were removed. Skin samples were processed as described previously [16]. Briefly, tumour samples were separated and placed in RPMI 1640 medium (Gibco, Grand Island, NY, USA) supplemented with 250 µg/mL collagenase (Boehringer Ingelheim Chemicals), 3000 U/mL DNase, 100 U/mL penicillin and 100 µg/ml streptomycin for 40 min at 37 °C. One cycle of cellular dissociation was performed for 4 min using a Medimachine (BD Biosciences). Cells were passed through a nylon mesh (30-µm pore size) and then processed for flow cytometry as described below. Tumour-draining lymph nodes were removed, and single-cell suspensions were obtained by passing the cells through a 70-µm cell strainer and prepared for flow cytometry. Surface and intracellular staining and flow cytometry were performed as described previously [14]. For intracellular staining, cells were fixed in 4% formaldehyde and then permeabilised using the Perm/Wash™ buffer kit (BD Biosciences), followed by incubation with antibodies. The following antibodies were used: anti-mouse CD19 (1D3), anti-mouse CD3 (145-2C11), anti-mouse CD4 PERCP (RM4-5), anti-mouse CD8 APC (53 − 6.7), anti-mouse IFNy PE (B27), anti-mouse IL-10 FITC (JES5-16E3) and anti-mouse IL-4-PE (11B11); the respective goat and rat isotype controls were used for each analysed antibody (BD Biosciences). Data were collected using an FACSCalibur (BD Immunocytometry Systems) and analysed using CellQuest software (BD Biosciences). Haematoxylin and eosin–stained sections were reviewed by two pathologists to confirm the histopathological diagnosis. Formalin-fixed and paraffin-embedded samples were collected from each tumour specimen. All sections were analysed under an optical microscope, and microphotographs were collected using a digital camera (Leica DFC310 FX, Leica Microsystems GmbH, Wetzlar, Germany). Representative sections from each lesion were used for histopathological analysis. Statistical significance was determined with Student’s t-test when comparing two groups or one-way analysis of variance (ANOVA) when comparing three or more groups. The log-rank (Mantel–Cox) test was used to determine whether the anti-IL-33-treated mice had better outcomes than the wild-type mice. The data are presented as the mean ± standard error of the mean (SEM) or the mean ± standard deviation (SD). A P value ≤ 0.05 was considered statistically significant. The Prism 8.3 software program (GraphPad Software, San Diego, CA, USA) was used for statical analysis.Antibodies and treatment protocol
Isolation of leucocytes
Flow cytometry
Histological analysis
Statistical analysis
Results
IL-33 expression in human SCC lesions
IL-33 is associated with a mouse model of SCC development [10]; therefore, we investigated which cells may be the source of IL-33 in the tumour microenvironment in human SCC lesions. We analysed the IL-33 protein levels in paraffin-embedded tissue sections of SCC (Fig. 1). We detected IL-33 in the peritumoural stroma of nearly all tissue sections (Fig. 1a). The IL-33+ cells in SCC tissue sections showed typical cytoplasmic staining; they were epithelial and mononuclear cells (Fig. 1a). We detected IL-33+ cells with mononuclear cell morphology. Overall, there were fewer IL-33+ peritumoural cells than IL-33+ epithelial cells (Fig. 1b and c).
Interleukin 33 (IL-33) in squamous cell carcinoma (SCC). (a) Representative micrographs (scale bar = 200 μm) of three patients with SCC, including haematoxylin and eosin staining (first line) and immunohistochemical staining for IL-33 (second line). (b) The graph represents the mean ± standard deviation (SD) of the percentage of IL-33+ peritumoural cells. (c) The graph represents the mean ± SD of the number of IL-33+ epithelial cells. (d) Relative expression of ST2 and IL33 determined by quantitative polymerase chain reaction (mean ± standard error of the mean, n = 3) in SCC cell lines
We also performed RT-qPCR to analyse IL33 and ST2 gene expression in two HNSCC cell lines. Detroit 562 cells were isolated from the pharynx of a patients with pharyngeal cancer and SCC-25 cells were isolated from the tongue of a patient with SCC. IL-33 and ST2 gene expression were not different between these two SCC lines (Fig. 1d). There were low IL-33 and ST2 mRNA levels in these cells.
Effects of IL-33 on the motility, proliferation and invasiveness of SCC-25 and Detroit 562 cells
IL-33 has a well-known impact on immune cells, especially Th2 cells in allergic disease and parasitic infections. However, in cancer immunity IL-33 can display both pro- and anti-tumoural functions, depending on the specific microenvironment. We evaluated the effect of IL-33 on the biology of SCC-25 and Detroit 562 cells, including their motility and growth. Our initial analysis with IncuCyte technology showed a significant dose-dependent effect of rhIL-33 on the migratory capacity of SCC-25 cells. Compared with the control, the migratory capacity of SCC-25 cells increased by 92.6% ± 3.4% (10 ng/mL rhIL-33), 80.7% ± 22.5% (50 ng/mL rhIL-33) and 83.0% ± 23.2% (100 ng/mL rhIL-33) (Fig. 2a and b). rhIL-33 treatment of SCC-25 cells also induced a significant dose-dependent increase in proliferation (Fig. 2b); 50 ng/mL rhIL-33 produced the maximum effect. We next analysed the effect of IL-33 on the biology of Detroit 562 cells, which are cells derived from metastatic sites [ Proposed model of the role of interleukin 33 (IL-33) in squamous cell carcinoma (SCC) growth via a dual effect on tumour proliferation, migration and invasion, and T cell activation. IL-33 + epithelial and mononuclear cells are found in in the SCC lesions (Fig. 1). The release of this cytokine induces changes in the biology of SCC cells by promoting a pro-tumour phenotype directly (Fig. 2) or indirectly by inducing other factors in the tumour microenvironment (not tested). The neutralisation of IL-33 restricts SCC outgrowth, decreases Th2-derived IL-4 and increases accumulation of Th1 IFNγ-producing cells in tumour-draining lymph nodes (Fig. 3), and thus represents an effective and promising strategy for SCC treatment. Created with Biorender