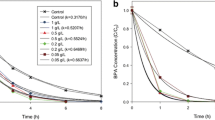
Abstract
DFT calculations in both gaseous phase and solution are carried out to investigate the degradation mechanism of tetrabromobisphenol A (TBBPA) under photocatalytic UV/Fenton conditions. It is found that there exist reductive process and oxidative process. Our calculations show that the reductive process is caused by a conduction band (CB) photoelectron e −CB to make the C–Br bond broken, while the oxidative process is due to ·OH radical attacking three possible sites of TBBPA to form different intermediates. In the reductive process, the reduction of TBBPA by a photoelectron e −CB is coupled with C–Br bond cleavage, and the formation of tribromobisphenol A radical (IM3) is the rate-determining step to form the reduction product tribromobisphenol A (P1), where in the experiment (Zhong et al. Water Res 46(15):4633–4644, 2012), mechanism being proposed as ·OH radical attacking C–Br bond. In the oxidative process, abstracting hydrogen atom by ·OH radical is the most plausible reaction to form 2,2-bis(3,5-dibromo-4-hydroxyphenyl)propane radical (IM4). IM4 can receive a conduction band electron e −CB to yield 2,2-bis(3,5-dibromo-4-hydroxyphenyl)propane carbanion (IM4′), followed by a C–C bond breaking reaction, resulting in the formation of P2 and 3,5-dibromophenol carbanion (IM5′).











Similar content being viewed by others
References
Kitamura S, **no N, Ohta S, Kuroki H, Fujimoto N (2002) Biochem Biophys Res Commun 293(1):554–559
Riess M, van Eldik R (1998) J Chromatogr A 827(1):65–71
Alaee M, Arias P, Sjödin A, Bergman Å (2003) Environ Int 29(6):683–689
Kitamura S, Suzuki T, Sanoh S, Kohta R, **no N, Sugihara K, Yoshihara S, Fujimoto N, Watanabe H, Ohta S (2005) Toxicol Sci 84(2):249–259
Abdallah MAE, Harrad S, Covaci A (2008) Environ Sci Technol 42(18):6855–6861
Luo XJ, Zhang XL, Liu J, Wu JP, Luo Y, Chen SJ, Mai BX, Yang ZY (2009) Environ Sci Technol 43(2):306–311
McKinney MA, Cesh LS, Elliott JE, Williams TD, Garcelon DK, Letcher RJ (2006) Environ Sci Technol 40(20):6275–6281
Meerts I, van Zanden JJ, Luijks EAC, van Leeuwen-Bol I, Marsh G, Jakobsson E, Bergman A, Brouwer A (2000) Toxicol Sci 56(1):95–104
Brenner A, Mukmenev I, Abeliovich A, Kushmaro A (2006) Ecotoxicology 15(4):399–402
Gerecke AC, Giger W, Hartmann PC, Heeb NV, Kohler HPE, Schmid P, Zennegg M, Kohler M (2006) Chemosphere 64(2):311–317
George KW, Haggblom MM (2008) Environ Sci Technol 42(15):5555–5561
Uhnáková B, Ludwig R, Pěknicová J, Homolka L, Lisá L, Šulc M, Petříčkova A, Elzeinová F, Pelantová H, Monti D, Křen V, Haltrich D, Martínková L (2011) Bioresour Technol 102(20):9409–9415
Peng XX, Zhang ZL, Luo WS, Jia XS (2013) Bioresour Technol 128:173–179
Ronen Z, Abeliovich A (2000) Appl Environ Microbiol 66(6):2372–2377
Mackenzie K, Kopinke FD (1996) Chemosphere 33(12):2423–2430
Lin KD, Ding JF, Huang XW (2012) Ind Eng Chem Res 51(25):8378–8385
Liu GB, Zhao HY, Thiemann T (2009) J Hazard Mater 169(1–3):1150–1153
Liu GB, Dai L, Gao X, Li MK, Thiemann T (2006) Green Chem 8(9):781–783
Luo S, Yang SG, Wang XD, Sun C (2012) Environ Eng Sci 29(6):453–460
Lin K, Liu WP, Gan J (2009) Environ Sci Technol 43(12):4480–4486
Zhu QQ, Mizutani Y, Maeno S, Fukushima M (2013) Molecules 18(5):5360–5372
Zhang KL, Huang J, Zhang W, Yu YF, Deng SB, Yu G (2012) J Hazard Mater 243:278–285
Monserrate E, Haggblom MM (1997) Appl Environ Microbiol 63(10):3911–3915
Xu J, Meng W, Zhang Y, Li L, Guo CS (2011) Appl Catal B-Environ 107(3–4):355–362
Sun CY, Chang W, Ma WH, Chen CC, Zhao JC (2013) Environ Sci Technol 47:2370–2377
Guo YG, Lou XY, **ao DX, Xu L, Wang ZH, Liu JS (2012) J Hazard Mater 241:301–306
He HY, Zapol P, Curtiss LA (2012) Energy Environ Sci 5(3):6196
Lee D, Kanai Y (2012) J Am Chem Soc 134(50):20266–20269
Chen JX, Zhu LZ (2007) J Photochem Photobiol A 188(1):56–64
De Laat J, Gallard H (1999) Environ Sci Technol 33(16):2726–2732
Tryba B, Piszcz M, Grzmil B, Pattek-Janczyk A, Morawski AW (2009) J Hazard Mater 162(1):111–119
Utset B, Garcia J, Casado J, Domènech X, Peral J (2000) Chemosphere 41:1187–1192
Pérez M, Torrades F, Domènech X, Peral J (2002) Water Res 36(11):2703–2710
Zhong YH, Liang XL, Zhong Y, Zhu JX, Zhu SY, Yuan P, He HP, Zhang J (2012) Water Res 46(15):4633–4644
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, F. IA, Bliino J, Zheng G, Sonnenberg JL, Hada M, Ehara KT, Fukuda R, Hasegawa J, Ishida M, T. N, Y. HOK, Nakai H, Vreven T, Montgomery JA Jr., Peralta JE, Oliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Straroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari KR, Jendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09. B.01 edn. Gaussian, Inc., Wallingford CT
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Miehlich B, Savin A, Stoll H, Preuss H (1989) Chem Phys Lett 157:200–206
Fukui K (1981) Acc Chem Res 14:363–368
Mraenich AV, Cramer CJ, Truhlar DG (2009) J Phys Chem B 113:6378–6396
Wu D, Song K, Fang Z, Gu FL (2013) Curr Phys Chem 3:179–186
Rotko G, Romańczyk PP, Andryianau G, Kurek SS (2014) Electrochem Commun 43:117–120
Brusa MA, Grela MA (2005) J Phys Chem B 109:1914–1918
Goldstein S, Meyerstein D (1999) Acc Chem Res 32:547–550
Maldotti A, Molinari A, Amadelli R, Carbonell E, Garcia H (2008) Photochem Photobiol Sci 7(7):819–825
Carneiro JT, Almeida AR, Moulijn JA, Mul G (2010) Phys Chem Chem Phys 12(11):2744–2750
Acknowledgments
The supports of the National Natural Science Foundation of China (21273081, 21073067) are greatly appreciated. Financial support from the Project supported by Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, X., Peng, L., Li, S. et al. Theoretical study on the sequential reduction and oxidation mechanism for tetrabromobisphenol A degradation under photocatalytic UV/Fenton conditions. Theor Chem Acc 134, 3 (2015). https://doi.org/10.1007/s00214-014-1604-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-014-1604-4