Abstract
The effect of vertebral osteoporosis on disc degeneration is still debated. The purpose of this study was to provide a systematic review of studies in this area to further reveal the relationship between the two. Relevant studies were searched in electronic databases, and studies were screened according to inclusion and exclusion criteria, and finally, basic information of the included studies was extracted and summarized. This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. A total of 34 publications spanning 24 years were included in our study. There were 19 clinical studies, including 12 prospective studies and 7 retrospective studies. Of these, 7 considered vertebral osteoporosis to be positively correlated with disc degeneration, 8 considered them to be negatively correlated, and 4 considered them to be uncorrelated. Two cadaveric studies were included, one considered the two to be negatively correlated and one considered them not to be correlated. Seven animal studies were included, of which five considered a positive correlation between vertebral osteoporosis and disc degeneration and two considered a negative correlation between the two. There were also 6 studies that used anti-osteoporosis drugs for intervention, all of them were animal studies. Five of them concluded that vertebral osteoporosis was positively associated with disc degeneration, and the remaining one concluded that there was no correlation between the two. Our systematic review shows that the majority of studies currently consider an association between vertebral osteoporosis and disc degeneration, but there is still a huge disagreement whether this association is positive or negative. Differences in observation time and follow-up time may be one of the reasons for the disagreement. A large number of clinical and basic studies are still needed in the future to further explore the relationship between the two.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intervertebral disc degeneration is a pathological change that occurs when the lumbar disc is damaged or ages with age. It can be induced by a variety of factors, such as mechanical loading, genetics, obesity, smoking, and aging. Although the mechanism remains unclear, degeneration and death of disc cells due to reduced nutrient supply, imbalance in microenvironmental homeostasis, infiltration of inflammatory factors, and altered mechanical loading are its main causes [4,5,6]. Another view is the exact opposite, that is, that a less dense vertebral body accelerates disc degeneration, while a healthy vertebral bone structure protects the disc [7,8,9]. Still others believe that the bone density of the vertebral body does not correlate with disc degeneration [10, 11].
A recent study based on quantitative Dixon and GRAPPATINI T2 map** techniques [4] found that disc degeneration caused by mechanical stress or aging often first manifests as loss of annulus fibrosus integrity, whereas the effect of osteoporosis on the lumbar disc is concentrated in the nucleus pulposus rather than the annulus fibrosus, suggesting that osteoporosis may directly trigger nucleus pulposus degeneration through the longitudinal structures (vertebral body-osseous endplates-bone marrow contact channels—cartilage endplates—nucleus pulposus), a pathological process completely different from disc degeneration caused by mechanical stress or aging.
Therefore, we believe that starting from the alteration of vertebral bone structure may provide a deeper understanding of the mechanisms of disc degeneration and may even lead to a potential means of intervention in the future. Over the last 20 years, there have been numerous clinical and animal studies to investigate the correlation between vertebral osteoporosis and disc degeneration. Unfortunately, no one has yet performed a systematic review of these studies. We believe that a systematic summary and evaluation of these studies is necessary. On the one hand, it helps one to understand the current research status in the field, and on the other hand, it helps to identify some shortcomings in the current research, which can provide guidance for future research design. Moreover, we may be able to find some common patterns from the existing research results, which can help to provide new ideas and entry points for future research.
Methods
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [12].
Literature search strategy and selection criteria
We conducted a literature search using the following search terms: osteoporosis, bone density, intervertebral disc degeneration, and their combined forms in several electronic databases, PubMed(1966 to January 1, 2023), Cochrane Library (1966 to January 1, 2023), and Embase (1980 to January 1, 2023). Target articles were identified step by step based on title, abstract, and full text.
Ultimately, studies that met the following criteria were included: (1) studies exploring the relationship between vertebral osteoporosis or reduced bone density and intervertebral discs or endplates, including clinical studies and animal studies, with no restriction on the level of evidence, country and language; (2) clinical studies could be prospective or retrospective. There was no restriction on the duration of observation and the imaging means used; (3) there was also no restriction on the type of animals, strain, modelling method, intervention method, or observation time. Reviews, commentaries, letters to the editor, unpublished articles, and retracted articles were excluded. For studies that met the inclusion criteria, we also traced their references to identify potential studies. Also, some high-quality reviews were important references and basis for this study. The literature search and screening was conducted independently by two researchers. Any disagreements that arose during that process were resolved through discussion.
Data extraction
According to the purpose of our study, we extracted the following information from the included studies: first author, year of publication, type of study, imaging method and indicator, type of animal, modeling method, intervention method, observation time, results, and conclusions. This work was done independently by two researchers.
Quality assessment of the literature
We evaluated the quality of the included retrospective and prospective clinical studies using the Newcastle–Ottawa Scale (NOS) and the quality of the included animal studies using the initial Stroke Therapy Academic Industry Roundtable list (STAIR).
Results
Literature search results
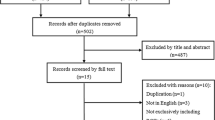
According to the search strategy, we obtained a total of 114 relevant studies, and 83 after removing duplicates. After screening by title and abstract, a total of 35 papers were excluded. The remaining 48 studies were evaluated in full text, 14 of which were excluded because they did not meet the inclusion criteria, and 34 studies were finally included. The literature screening process is shown in Fig. 1. Basic information on the included clinical studies is shown in Table 1, basic information on the included animal studies is shown in Table 2, and basic information on the included drug therapy-related studies is shown in Table 3. Of the 34 studies, 17 considered positive correlation between vertebral osteoporosis and disc degeneration, 11 considered negative correlation, and 6 considered no correlation.
Clinical and cadaveric studies
We included a total of 21 clinical and cadaveric studies, including 12 prospective studies, 7 retrospective studies, and 2 cadaveric studies. Among the clinical studies, 7 studies considered vertebral osteoporosis to be positively associated with disc degeneration, 8 studies considered them to be negatively associated, and 4 studies considered them not to be associated. Of the cadaveric studies, 1 concluded that they were negatively correlated and 1 concluded that they were not correlated. The earliest of these studies was published in 1998 [21] and the most recent in 2022 [7]. The largest sample size was the study by Livshits et al. [16] and the smallest sample size was the study by Tosun et al. [6]. The middle-aged and older groups of men and women were the main subjects of clinical studies. In terms of imaging tools, the most used tool for measuring bone mineral density was dual-energy X-ray absorptiometry (DEXA), while some studies also used quantitative computed tomography (QCT). One study [14] used the dynamic computed tomographic perfusion (CTP) technique to detect indicators related to bone marrow microcirculation perfusion, and two other studies [7, 9] used Hounsfield Unit (HU) values from computed tomography (CT) instead of bone density. Magnetic resonance imaging (MRI) is a widely used tool to assess disc degeneration. Its indices are more diverse and include Pfirrmann grading (the most used), intervertebral space height, degree of endplate damage, bone redundancy formation, disc volume, and disc bulge rate. Two cadaveric studies [22, 25] used discography to assess the degree of disc degeneration and Micro-CT to measure bone density and endplate related parameters. With regard to the timing of assessment, most studies performed only one assessment of bone density and disc at sample entry, and only Salo et al. [23] performed three follow-up visits 5, 10, and 15 years after entry.
Animal studies
We included a total of 7 animal studies, of which 5 concluded that vertebral osteoporosis was positively associated with disc degeneration and 2 concluded that they were negatively associated. The earliest of these animal studies was published in 2004 [32] and the most recent study [30] combined paravertebral muscle dissection with ovariectomy. In terms of observation time, Zhang et al. [4, 5, and 6.
CT computed tomography, HU Hounsfield Unit, MRI magnetic resonance imaging, TEPS total endplate score, DEXA dual-energy X-ray absorptiometry, BMD bone mineral density, CTP dynamic computed tomographic perfusion, Ha anterior height, Hm middle height, Hp posterior height, AP anteriorposterior dimension, MZ monozygotic, DZ dizygotic, LDD lumbar disc degeneration, QCT quantitative computed tomography, Q-Dixon quantitative Dixon, Int.BMD integral BMD, Tb.BMD trabecular BMD, Trab.vBMD trabecular volumetric BMD, M male, F female, BMC bone mineral content, BV bone volume, TV total volume, BV/TV percentage bone volume, BS bone surface density, Tb.N trabecular number, Tb.Th trabecular thickness, Tb.Sp trabecular separation, SMI structural model index, BMDtv total volume of bone mineral density, Tb.Pf trabecular pattern factor, CONN.D connectivity density, Po.N(cl) number of closed pores, PO(op) open porosity, Po.V(tot) total volume of pore space, Mdtv mean density of TV, DHI disc height index, IDH intervertebral disc height, VEL vertebral endplates lesions, SD Sprague Dawley, OVX ovariectomy, Opg osteoprotegerin, KO knockout, VEGF-A vascular endothelial growth factor-A, IL-1β interleukin-1β, TNF-α tumor necrosis factor-α, PYM **yangmycin, CMS cervical muscle section.
Discussion
The physiological and pathological characteristics of intervertebral discs, such as their anatomical structure, nutritional pathways, and mechanical properties, have been studied quite thoroughly as early as the last century. In recent years, although clinical and basic research on discs is still gradually increasing, there seems to be few groundbreaking findings. For disc degeneration, there is also still no recognized effective intervention and remains one of the most urgent challenges to be solved. When it comes to vertebral osteoporosis, more attention is often paid to the vertebral fractures caused by it and its impact on lumbar spine surgery, such as on the stability of intervertebral fusion devices and pedicle screws. There are not many studies examining the effects of vertebral osteoporosis on disc degeneration, and the findings are divergent. Therefore, we designed this study. To our knowledge, this is the first time in the last two decades that a systematic and comprehensive review and summary of relevant studies has been performed. This helps one to understand the points of controversy and the problems with the current study, so that the next studies can be improved in a targeted manner.
In this systematic review, we have made a comprehensive and detailed summary and presentation of the basic information of 34 studies, such as study type, sample information, imaging methods, modeling methods, observation time, results, and conclusions, and we have also evaluated the quality of these studies. Of the 34 studies, 17 concluded that vertebral osteoporosis was positively correlated with disc degeneration, 11 concluded that they were negatively correlated, and 6 concluded that they were not correlated. Most of the retrospective clinical studies we included were cross-sectional studies, which had a high risk of bias and a limited level of evidence. Prospective studies were more convincing than retrospective studies, but these studies lacked long follow-up. Moreover, the included clinical studies commonly used DEXA to measure BMD, and their results are susceptible to interference from other tissues [20]. In addition, these clinical studies could not be explored in greater depth at the level of mechanism of action. The animal studies we included were small in number and each had a small sample size, but they provided a wealth of histological evidence in addition to imaging findings. Moreover, these animal studies commonly used Micro-CT to measure bone trabecular structures as well as endplate microstructure, which is more accurate than DEXA. Encouragingly, several studies [61] concluded that the rate of glucose diffusion into the disc significantly decreases as the static compressive strain of the disc increases. When the vertebral bone density decreases, the endplate resistance decreases and the compressive strain of the disc decreases, which facilitates the diffusion of glucose and delays the degeneration of the disc.
Contrary to the above, other evidence suggests that the vertebral body after osteoporosis does not act as a stress buffer, but rather increases the mechanical stresses on the endplate and disc. Finite element models [73] showed that when the modulus of trabeculae decreased by 50%, the peak stress on the endplate increased by 74%, eventually leading to endplate thinning, calcification [74], and even collapse [25, 75, 76]. Histological evidence also suggests the presence of many active small chondrocytes within the cartilage endplate adjacent to the nucleus pulposus, which is considered an indication of abnormal endplate loading [77]. The overgrowth of bony flaps at the edges of the vertebral body in osteoporotic rats is also an evidence of increased disc stress [78]. We believe that the force patterns of the lumbar spine are different in humans and experimental animals. Humans walk upright, whereas experimental animals are crawlers. Therefore, clinical studies may reach different conclusions than animal studies, which may be one of the reasons for the disagreement. In addition, the difference in observation time between the above studies may also contribute to the disagreement. Bone loss is a long-term process. In the early stages, a mild decrease in vertebral bone density may serve to cushion the pressure to protect the disc, but this cushioning effect may be difficult to maintain as the bone structure gradually deteriorates. Unfortunately, no one has yet observed the dynamic effects of vertebral bone density on the disc at different times. Therefore, this question still needs to be answered by more future studies.
Disc height
Changes in disc height after reduction in vertebral bone density were observed in several animal experiments and clinical studies in our included studies [6, 15, 17, 24]. Exceptionally, disc height changes under osteoporosis are more often reflected in an increase in the intermediate disc height [6, 24], whereas the anterior and posterior disc heights do not seem to change significantly [24]. Biomechanical studies have shown that osteoporosis leads to a concentration of stress in the center of the vertebral endplate, and the increased pressure leads to trabecular compression and axial bulging of the endplate [79]. This is therefore manifested on imaging as an increased concavity of the endplate [24], leading to an increase in the intermediate height of the disc. However, it is important to point out that this is a secondary change due to the additional expansion space created by the increased concavity of the endplate and the compression of the vertebral body, and is not a change in the state of the disc itself. It is also believed a loss of vertebral body height due to osteoporosis causes instability of the spinal motion segment and accelerates degeneration of the articular eminence joint and disc [17, 80]. We suggest that when exploring disc height, humans and animals similarly show different results depending on different walking patterns. Changes in disc height may be more pronounced in humans who walk upright relative to animals who crawl. Therefore, the plausibility of using animal disc height changes to model human disc degeneration seems to be debatable.
Imaging tools
DEXA is the most widely used method for screening and diagnosis of osteoporosis. It has the advantage of being technically mature, easy to perform, and inexpensive, but it has limitations in terms of spatial resolution and is susceptible to interference from endplate sclerosis, bone redundancy, and ligamentous calcification, leading to falsely elevated measurements [20]. Therefore, it has been suggested [23, 26] that the conclusion that higher BMD is associated with more severe disc degeneration may be related to the falsely elevated BMD values caused by DEXA. Furthermore, DEXA does not allow for the quantitative analysis of bone tissue in the target region. In view of this limitation, more and more studies have started to use Q-CT and Micro-CT to quantify the microstructure of bone trabeculae in the target region after differentiating between cortical and cancellous bone to reduce the interference of other structures. Micro-CT can clearly scan bone trabeculae in the target area at the micron level, calculate bone structural parameters precisely and in three dimensions, and detect early changes in bone microstructure. Some impressive studies [25, 29] used Micro-CT to precisely delineate the target area, segment the endplate, and directly measure the calcified area, thickness, and porosity of the endplate to observe structural changes in the endplate from a more microscopic perspective. However, Micro-CT is not as popular as DEXA due to its high price, complicated operation, and high radiation dose, and is currently used more for basic research. There is also an alternative method of assessing BMD using the HU value, which is based on the existence of a strong positive correlation between HU values and BMD [9]. As an alternative method, no additional examination costs are required and the procedure is relatively simple. In the previously mentioned section on bone marrow perfusion, functional CTP imaging has been used to quantify the spatio-temporal distribution patterns of bone marrow microcirculation perfusion and bone density, which is also an emerging technical tool for the assessment of hemodynamics by continuous measurement of multiplanar imaging [14]. As for the assessment of disc degeneration, it is evident from the included studies that MRI is still the most commonly used imaging method to assess the disc.
Animal models of osteoporosis
Cost is an important factor influencing the choice of experimental animals. In our included studies, rats and mice remained the most used animals due to their lower cost. Primates share anatomical, physiological and biomechanical similarities with humans and are higher up in the phylogenetic tree. Of the studies we included, only one study [29] used rhesus monkeys for the study. We believe that large-scale use of primates may remain elusive due to high costs. Accelerated bone turnover due to estrogen deficiency is the most common cause of osteoporosis. Therefore, the animal model of osteoporosis caused by estrogen deficiency is the most used animal model in our included studies. This model was constructed by bilateral oophorectomy, which is a mature technique, simple to perform, and has a high modeling rate. Other studies have used models of natural aging, which have a longer experimental period and higher time cost. OPG exerts an inhibitory effect on the differentiation, activation and survival of osteoblasts. One study [28] used a mouse model with OPG knockout, again limited by cost and difficult to apply on a large scale. Other studies have used composite models, which add to the OVX model by removing paravertebral muscles to exacerbate lumbar instability [30] or by injecting drugs into the endplate to block the nutrient supply to the disc [29], achieving a more significant disc degeneration effect than the OVX model alone and shortening the experimental period.
Limitations
We have the following limitations in this study. First, the included studies were highly variable with few common outcome indicators, so we were unable to perform a quantitative meta-analysis of the valuable outcome indicators to increase the persuasiveness. Second, because disc degeneration is the result of a combination of factors and there is no ideal experimental model to date that can model any one of these factors alone [14]. Therefore, the studies we included only illustrate the correlation between vertebral osteoporosis and disc degeneration without revealing a causal relationship between the two. In addition, the relationship between vertebral osteoporosis and disc degeneration may not be one-sided, but rather an interactive relationship. Disc degeneration can likewise have an impact on vertebral bone density. Some studies [6, 10, 23] have shown that when the disc degenerates, the load shifts from the nucleus pulposus to the annulus fibrosus and the stress on the vertebral wall and posterior structures increases, leading to a decrease in core bone density and an increase in bone density in the vertebral wall and posterior structures. However, due to space limitations, we did not explore the effect of disc degeneration on vertebral bone density. Finally, osteoporosis is a systemic disease, and more in-depth studies on the relationship between vertebral and extremity bone density, as well as the relationship between disc degeneration and extremity bone density, are still pending [16, 18, 81, 82], which is our next step to focus on.
Conclusion
Our systematic review shows that the majority of studies currently consider an association between vertebral osteoporosis and intervertebral disc degeneration, but there is still a huge disagreement whether this association is positive or negative. Differences in observation time and follow-up time may be one of the reasons for the disagreement. Our view is that vertebral osteoporosis may have a bidirectional effect on disc degeneration. At different stages in the progression of osteoporosis, the effect on disc degeneration shows different trends. Therefore, for future clinical and animal studies, we not only recommend the use of more precise imaging tools such as QCT and Micro-CT, but also the setting of different observation time points to explore the dynamic relationship between vertebral osteoporosis and disc degeneration.
Data availability
All data generated or analyzed during this study are included in this article.
References
**n J, Wang Y, Zheng Z, Wang S, Na S, Zhang S (2022) Treatment of intervertebral disc degeneration. Orthop Surg 14(7):1271–1280. https://doi.org/10.1111/os.13254
Miller JA, Schmatz C, Schultz AB (1988) Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine. 13:173–8
Bijlsma AY, Meskers CG, Westendorp RG, Maier AB (2012) Chronology of age-related disease definitions: osteoporosis and sarcopenia. Ageing Res Rev 11(2):320–324. https://doi.org/10.1016/j.arr.2012.01.001
Li X, ** parameters: a whole spinal assessment of the relationship between osteoporosis and intervertebral disc degeneration. J Magn Reson Imaging 55(5):1536–1546. https://doi.org/10.1002/jmri.27959
Kaiser J, Allaire B, Fein PM, Lu D, Jarraya M, Guermazi A, Demissie S, Samelson EJ, Bouxsein ML, Morgan EF (2018) Correspondence between bone mineral density and intervertebral disc degeneration across age and sex. Arch Osteoporos 13(1):123. https://doi.org/10.1007/s11657-018-0538-1
Tosun O, Fidan F, Erdil F, Tosun A, Karaoğlanoğlu M, Ardıçoğlu O (2012) Assessment of lumbar vertebrae morphology by magnetic resonance imaging in osteoporosis. Skeletal Radiol 41(12):1583–1590. https://doi.org/10.1007/s00256-012-1435-0
Liang X, Liu Q, Xu J, Ding W, Wang H (2022) Hounsfield unit for assessing bone mineral density distribution within cervical vertebrae and its correlation with the intervertebral disc degeneration. Front Endocrinol 13:920167. https://doi.org/10.3389/fendo.2022.920167
Zhao Y, Wang H, Li Z, Wang Z, Huo Y, Yang S, Ding W (2021) Lumbar disk degeneration in female patients with and without ovariectomy: a case-control study. World Neurosurg 156:68–75. https://doi.org/10.1016/j.wneu.2021.09.080
Zhuang C, Wang Z, Chen W, Tian B, Li J, Lin H (2021) Osteoporosis and endplate damage correlation using a combined approach of hounsfield unit values and total endplate scores: a retrospective cross-sectional study. Clin Interv Aging 5(16):1275–1283. https://doi.org/10.2147/CIA.S315213
Geng J, Wang L, Li Q, Huang P, Liu Y, Blake GM, Tian W, Cheng X (2021) The association of lumbar disc herniation with lumbar volumetric bone mineral density in a cross-sectional Chinese study. Diagnostics 11(6):938. https://doi.org/10.3390/diagnostics11060938
Pan J, Lu X, Yang G, Han Y, Tong X, Wang Y (2017) Lumbar disc degeneration was not related to spine and hip bone mineral densities in Chinese: facet joint osteoarthritis may confound the association. Arch Osteoporos 12(1):20. https://doi.org/10.1007/s11657-017-0315-6
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 3(3):123–30
Fabreguet I, Fechtenbaum J, Briot K, Paternotte S, Roux C (2013) Lumbar disc degeneration in osteoporotic men: prevalence and assessment of the relation with presence of vertebral fracture. J Rheumatol 40(7):1183–1190. https://doi.org/10.3899/jrheum.120769
Ou-Yang L, Lu GM (2015) Dysfunctional microcirculation of the lumbar vertebral marrow prior to the bone loss and intervertebral discal degeneration. Spine 40(10):593–600. https://doi.org/10.1097/BRS.0000000000000834
Kwok AW, Wang YX, Griffith JF, Deng M, Leung JC, Ahuja AT, Leung PC (2012) Morphological changes of lumbar vertebral bodies and intervertebral discs associated with decrease in bone mineral density of the spine: a cross-sectional study in elderly subjects. Spine 37(23):1415–21. https://doi.org/10.1097/BRS.0b013e31826f561e
Livshits G, Ermakov S, Popham M, Macgregor AJ, Sambrook PN, Spector TD, Williams FM (2010) Evidence that bone mineral density plays a role in degenerative disc disease: the UK Twin Spine study. Ann Rheum Dis 69(12):2102–2106. https://doi.org/10.1136/ard.2010.131441
Wang YX, Griffith JF, Ma HT, Kwok AW, Leung JC, Yeung DK, Ahuja AT, Leung PC (2011) Relationship between gender, bone mineral density, and disc degeneration in the lumbar spine: a study in elderly subjects using an eight-level MRI-based disc degeneration grading system. Osteoporos Int 22(1):91–96. https://doi.org/10.1007/s00198-010-1200-y
Pye SR, Reid DM, Adams JE, Silman AJ, O’Neill TW (2006) Radiographic features of lumbar disc degeneration and bone mineral density in men and women. Ann Rheum Dis 65(2):234–238. https://doi.org/10.1136/ard.2005.038224
Nanjo Y, Morio Y, Nagashima H, Hagino H, Teshima R (2003) Correlation between bone mineral density and intervertebral disk degeneration in pre- and postmenopausal women. J Bone Miner Metab 21(1):22–27. https://doi.org/10.1007/s007740300004
Miyakoshi N, Itoi E, Murai H, Wakabayashi I, Ito H, Minato T (2003) Inverse relation between osteoporosis and spondylosis in postmenopausal women as evaluated by bone mineral density and semiquantitative scoring of spinal degeneration. Spine 28(5):492–5. https://doi.org/10.1097/01.BRS.0000048650.39042.58
Harada A, Okuizumi H, Miyagi N, Genda E (1998) Correlation between bone mineral density and intervertebral disc degeneration. Spine 23(8):857–61. https://doi.org/10.1097/00007632-199804150-00003
Wang Y, Boyd SK, Battié MC, Yasui Y, Videman T (2011) Is greater lumbar vertebral BMD associated with more disk degeneration? A study using µCT and discography. J Bone Miner Res 26(11):2785–2791. https://doi.org/10.1002/jbmr.476
Salo S, Leinonen V, Rikkonen T, Vainio P, Marttila J, Honkanen R, Tuppurainen M, Kröger H, Sirola J (2014) Association between bone mineral density and lumbar disc degeneration. Maturitas 79(4):449–455. https://doi.org/10.1016/j.maturitas.2014.09.003
Yang Z, Griffith JF, Leung PC, Lee R (2009) Effect of osteoporosis on morphology and mobility of the lumbar spine. Spine 34(3):115–21. https://doi.org/10.1097/BRS.0b013e3181895aca
Wang Y, Battié MC, Boyd SK, Videman T (2011) The osseous endplates in lumbar vertebrae: thickness, bone mineral density and their associations with age and disk degeneration. Bone 48(4):804–809. https://doi.org/10.1016/j.bone.2010.12.005
**ao ZF, Su GY, Hou Y, Chen SD, Zhao BD, He JB, Zhang JH, Chen YJ, Lin DK (2020) Mechanics and biology interact in intervertebral disc degeneration: a novel composite mouse model. Calcif Tissue Int 106(4):401–414. https://doi.org/10.1007/s00223-019-00644-8
**ao ZF, He JB, Su GY, Chen MH, Hou Y, Chen SD, Lin DK (2018) Osteoporosis of the vertebra and osteochondral remodeling of the endplate causes intervertebral disc degeneration in ovariectomized mice. Arthritis Res Ther 20(1):207. https://doi.org/10.1186/s13075-018-1701-1
Li XF, Xue CC, Zhao YJ, Cheng SD, Zhao DF, Liang QQ, Chen L, Wang Q, Lu S, Shi Q, Wang YJ, Shu B (2017) Deletion of Opg leads to increased neovascularization and expression of inflammatory cytokines in the lumbar intervertebral disc of mice. Spine 42(1):8-E14. https://doi.org/10.1097/BRS.0000000000001701
Zhong R, Wei F, Wang L, Cui S, Chen N, Liu S, Zou X (2016) The effects of intervertebral disc degeneration combined with osteoporosis on vascularization and microarchitecture of the endplate in rhesus monkeys. Eur Spine J 25(9):2705–2715. https://doi.org/10.1007/s00586-016-4593-2
Ding Y, Jiang J, Zhou J, Wu X, Huang Z, Chen J, Zhu Q (2014) The effects of osteoporosis and disc degeneration on vertebral cartilage endplate lesions in rats. Eur Spine J 23(9):1848–1855. https://doi.org/10.1007/s00586-014-3324-9
Zhang Y, **a J, Qiu Y, Bai Y (2012) Correlation between osteoporosis and degeneration of intervertebral discs in aging rats. J Huazhong Univ Sci Technolog Med Sci 32(2):210–215. https://doi.org/10.1007/s11596-012-0037-3
Wang T, Zhang L, Huang C, Cheng AG, Dang GT (2004) Relationship between osteopenia and lumbar intervertebral disc degeneration in ovariectomized rats. Calcif Tissue Int 75(3):205–213. https://doi.org/10.1007/s00223-004-0240-8
Sun Q, Tian FM, Liu F, Fang JK, Hu YP, Lian QQ, Zhou Z, Zhang L (2021) Denosumab alleviates intervertebral disc degeneration adjacent to lumbar fusion by inhibiting endplate osteochondral remodeling and vertebral osteoporosis in ovariectomized rats. Arthritis Res Ther 23(1):152. https://doi.org/10.1186/s13075-021-02525-8
Luo Y, Zhang L, Wang WY, Hu QF, Song HP, Su YL, Zhang YZ (2013) Alendronate retards the progression of lumbar intervertebral disc degeneration in ovariectomized rats. Bone 55(2):439–448. https://doi.org/10.1016/j.bone.2013.03.002
Liu Q, Wang X, Hua Y, Kong G, Wu X, Huang Z, Huang Z, Liu J, Yang Z, Zhu Q (2019) Estrogen deficiency exacerbates intervertebral disc degeneration induced by spinal instability in rats. Spine 44(9):510–519. https://doi.org/10.1097/BRS.0000000000002904
Luo Y, Zhang L, Wang WY, Hu QF, Song HP, Zhang YZ (2015) The inhibitory effect of salmon calcitonin on intervertebral disc degeneration in an ovariectomized rat model. Eur Spine J 24(8):1691–1701. https://doi.org/10.1007/s00586-014-3611-5
Chen CH, Chen WC, Lin CY, Chen CH, Tsuang YH, Kuo YJ (2018) Sintered dicalcium pyrophosphate treatment attenuates estrogen deficiency-associated disc degeneration in ovariectomized rats. Drug Des Devel Ther 18(12):3033–3041. https://doi.org/10.2147/DDDT.S170816
Luo Y, Li SY, Tian FM, Song HP, Zhang YZ, Zhang L (2018) Effects of human parathyroid hormone 1–34 on bone loss and lumbar intervertebral disc degeneration in ovariectomized rats. Int Orthop 42(5):1183–1190. https://doi.org/10.1007/s00264-018-3821-2
Horner HA, Urban JP (2001) 2001 Volvo Award Winner in Basic Science Studies: Effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc. Spine 26(23):2543–9. https://doi.org/10.1097/00007632-200112010-00006
Nerlich AG, Schaaf R, Wälchli B, Boos N (2007) Temporo-spatial distribution of blood vessels in human lumbar intervertebral discs. Eur Spine J 16(4):547–555. https://doi.org/10.1007/s00586-006-0213-x
Grunhagen T, Wilde G, Soukane DM, Shirazi-Adl SA, Urban JP (2006) Nutrient supply and intervertebral disc metabolism. J Bone Joint Surg Am 88(2):30–35. https://doi.org/10.2106/JBJS.E.01290
Laffosse JM, Accadbled F, Molinier F, Bonnevialle N, de Gauzy JS, Swider P (2010) Correlations between effective permeability and marrow contact channels surface of vertebral endplates. J Orthop Res 28(9):1229–1234. https://doi.org/10.1002/jor.21137
Zhu Q, Gao X, Levene HB, Brown MD, Gu W (2016) Influences of nutrition supply and pathways on the degenerative patterns in human intervertebral disc. Spine 41(7):568–76. https://doi.org/10.1097/BRS.0000000000001292
Wu Y, Cisewski SE, Wegner N, Zhao S, Pellegrini VD Jr, Slate EH, Yao H (2016) Region and strain-dependent diffusivities of glucose and lactate in healthy human cartilage endplate. J Biomech 49(13):2756–2762. https://doi.org/10.1016/j.jbiomech.2016.06.008
Rodriguez AG, Rodriguez-Soto AE, Burghardt AJ et al (2012) Morphology of the human vertebral endplate. J Orthop Res 30(2):280–287
Zhang Y, Lenart BA, Lee JK et al (2014) Histological features of endplates of the mammalian spine: from mice to men. Spine 39(5):312–317
van der Werf M, Lezuo P, Maissen O, van Donkelaar CC, Ito K (2007) Inhibition of vertebral endplate perfusion results in decreased intervertebral disc intranuclear diffusive transport. J Anat 211(6):769–774. https://doi.org/10.1111/j.1469-7580.2007.00816.x
Jackson AR, Huang CY, Brown MD, Gu WY (2011) 3D finite element analysis of nutrient distributions and cell viability in the intervertebral disc: effects of deformation and degeneration. J Biomech Eng. 133(9):091006. https://doi.org/10.1115/1.4004944
Oki S, Matsuda Y, Shibata T et al (1996) Morphologic differences of the vascular buds in the vertebral endplate: scanning electron microscopic study. Spine 21(2):174–177
Benneker LM, Heini PF, Alini M, Anderson SE, Ito K (2005) 2004 Young Investigator Award Winner: vertebral endplate marrow contact channel occlusions and intervertebral disc degeneration. Spine 30(2):167–73. https://doi.org/10.1097/01.brs.0000150833.93248.09
Frost HM (1990) Skeletal structural adaptations to mechanical usage (SATMU): 2. Redefining Wolff’s law: the remodeling problem. Anat Rec. 226(4):414–22. https://doi.org/10.1002/ar.1092260403
Rodan GA, Martin TJ (2000) Therapeutic approaches to bone diseases. Science 289(5484):1508–1514. https://doi.org/10.1126/science.289.5484.1508
Hadjidakis DJ, Androulakis II (2006) Bone remodeling. Ann N Y Acad Sci 1092:385–396. https://doi.org/10.1196/annals.1365.035
Roberts S, Evans H, Trivedi J et al (2006) Histology and pathology of thehuman intervertebral disc. J Bone Joint Surg Am 88(2):10–14
Burr DB, Radin EL (2003) Microfractures and microcracks in subchondral bone: are they relevant to osteoarthrosis? Rheum Dis Clin North Am 29(4):675–685. https://doi.org/10.1016/s0889-857x(03)00061-9
Peng B, Chen J, Kuang Z, Li D, Pang X, Zhang X (2009) Expression and role of connective tissue growth factor in painful disc fibrosis and degeneration. Spine 34(5):178–82. https://doi.org/10.1097/BRS.0b013e3181908ab3
Ye F, Lyu FJ, Wang H, Zheng Z (2022) The involvement of immune system in intervertebral disc herniation and degeneration. JOR Spine 5(1):1196. https://doi.org/10.1002/jsp2.1196.。
Koike Y, Uzuki M, Kokubun S, Sawai T (2003) Angiogenesis and inflammatory cell infiltration in lumbar disc herniation. Spine 28(17):1928–33. https://doi.org/10.1097/01.BRS.0000083324.65405.AE
Tapia-Perez H (2008) Intervertebral disc pathologies from an immunologicalperspective. Revista de neurologia 46(12):751–7
Fujita N, Imai J, Suzuki T, Yamada M, Ninomiya K, Miyamoto K, Iwasaki R, Morioka H, Matsumoto M, Chiba K, Watanabe S, Suda T, Toyama Y, Miyamoto T (2008) Vascular endothelial growth factor-A is a survival factor for nucleus pulposus cells in the intervertebral disc. Biochem Biophys Res Commun 372(2):367–372. https://doi.org/10.1016/j.bbrc.2008.05.044
Seol D, Choe H, Zheng H et al (2011) Selection of reference genes fornormalization of quantitative real-time PCR in organ culture of therat and rabbit intervertebral disc. BMC Res Notes 4:162
Mattei TA (2013) Osteoporosis delays intervertebral disc degeneration by increasing intradiscal diffusive transport of nutrients through both mechanical and vascular pathophysiological pathways. Med Hypotheses 80(5):582–586. https://doi.org/10.1016/j.mehy.2013.01.030
Turgut M, Uslu S, Uysal A, Yurtseven ME, Ustün H (2003) Changes in vascularity of cartilage endplate of degenerated intervertebral discs in response to melatonin administration in rats. Neurosurg Rev 26(2):133–138. https://doi.org/10.1007/s10143-003-0259-8
Silverman NE, Nicklas BJ, Ryan AS (2009) Addition of aerobic exercise to aweight loss program increases BMD, with an associated reduction in in-flammation in overweight postmenopausal women. Calcif TissueInt 84:257–265
Armas LA, Recker RR (2012) Pathophysiology of osteoporosis: new mechanistic insights. Endocrinol Metab Clin North Am 41(3):475–486. https://doi.org/10.1016/j.ecl.2012.04.006
Kauppila LI, Penttila A, Karhunen PJ et al (1994) Lumbar disc degeneration and atherosclerosis of the abdominal aorta. Spine 19:923–929
Kurunlahti M, Tervonen O, Vanharanta H et al (1999) Association of atherosclerosis with low back pain and the degree of disc degeneration. Spine 24:2080–2084
Burkhardt R, Kettner G, Böhm W, Schmidmeier M, Schlag R, Frisch B, Mallmann B, Eisenmenger W, Gilg T (1987) Changes in trabecular bone, hematopoiesis and bone marrow vessels in aplastic anemia, primary osteoporosis, and old age: a comparative histomorphometric study. Bone 8(3):157–164. https://doi.org/10.1016/8756-3282(87)90015-9
Griffith JF, Yeung DK, Antonio GE, Lee FK, Hong AW, Wong SY, Lau EM, Leung PC (2005) Vertebral bone mineral density, marrow perfusion, and fat content in healthy men and men with osteoporosis: dynamic contrast-enhanced MR imaging and MR spectroscopy. Radiology 236(3):945–951. https://doi.org/10.1148/radiol.2363041425
Griffith JF, Yeung DK, Antonio GE, Wong SY, Kwok TC, Woo J, Leung PC (2006) Vertebral marrow fat content and diffusion and perfusion indexes in women with varying bone density: MR evaluation. Radiology 241(3):831–838. https://doi.org/10.1148/radiol.2413051858
Liu YJ, Huang GS, Juan CJ, Yao MS, Ho WP, Chan WP (2009) Intervertebral disk degeneration related to reduced vertebral marrow perfusion at dynamic contrast-enhanced MRI. AJR Am J Roentgenol 192(4):974–979. https://doi.org/10.2214/AJR.08.1597
Urban JP, Winlove CP (2007) Pathophysiology of the intervertebral disc and the challenges for MRI. J Magn Reson Imaging 25:419–432
Anthony W.L. Kwok; James F. Griffith; Heather Ting Ma; Yixiang Wang; ** Chung Leung; Jason Leung; David K.W. Yeung (2008). Estimated volume of both vertebral body and disc decreases as BMD decreases though this effect is seen more in the vertebral body than the disc 43 66. https://doi.org/10.1016/j.bone.2008.08.079
Mizrahi J, Silva MJ, Keaveny TM, Edwards WT, Hayes WC (1993) Finite-element stress analysis of the normal and osteoporotic lumbar vertebral body. Spine 18(14):2088–96. https://doi.org/10.1097/00007632-199310001-00028
Anderson DD, Brown TD, Radin EL (1993) The influence of basal cartilage calcification on dynamic juxtaarticular stress transmission. Clin Orthop Relat Res 286:298–307
Haschtmann D, Stoyanov JV, Gédet P, Ferguson SJ (2008) Vertebral endplate trauma induces disc cell apoptosis and promotes organ degeneration in vitro. Eur Spine J 17(2):289–299. https://doi.org/10.1007/s00586-007-0509-5
Wang Y, Videman T, Battié MC (2012) ISSLS prize winner: Lumbar vertebral endplate lesions: associations with disc degeneration and back pain history. Spine 37(17):1490–6. https://doi.org/10.1097/BRS.0b013e3182608ac4
Peng B, Shi Q, Shen P, Wang Y, Jia L (1999) The relationship between cartilage end-plate calcification and disc degeneration: an experimental study. Zhonghua Wai Ke Za Zhi 37(10):613–616
Kurowski P, Kubo A (1986) The relationship of degeneration of the intervertebral disc to mechanical loading conditions on lumbar vertebrae. Spine 11(7):726–31. https://doi.org/10.1097/00007632-198609000-00012
Ferguson SJ, Ito K, Nolte LP (2004) Fluid flow and convective transport of solutes within the intervertebral disc. J Biomech 37(2):213–221. https://doi.org/10.1016/s0021-9290(03)00250-1
Margulies JY, Payzer A, Nyska M, Neuwirth MG, Floman Y, Robin GC (1996) The relationship between degenerative changes and osteoporosis in the lumbar spine. Clin Orthop Relat Res 324:145–152. https://doi.org/10.1097/00003086-199603000-00017
Li R, Zhang W, Xu Y, Ma L, Li Z, Yang D, Ding W (2022) Vertebral endplate defects are associated with bone mineral density in lumbar degenerative disc disease. Eur Spine J 31(11):2935–2942. https://doi.org/10.1007/s00586-022-07329-1
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Li, W., Zhao, H., Zhou, S. et al. Does vertebral osteoporosis delay or accelerate lumbar disc degeneration? A systematic review. Osteoporos Int 34, 1983–2002 (2023). https://doi.org/10.1007/s00198-023-06880-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-023-06880-x