Abstract
Purpose
To evaluate whether helmet noninvasive ventilation compared to usual respiratory support reduces 180-day mortality and improves health-related quality of life (HRQoL) in patients with acute hypoxemic respiratory failure due to COVID-19 pneumonia.
Methods
This is a pre-planned follow-up study of the Helmet-COVID trial. In this multicenter, randomized clinical trial, adults with acute hypoxemic respiratory failure (n = 320) due to coronavirus disease 2019 (COVID-19) were randomized to receive helmet noninvasive ventilation or usual respiratory support. The modified intention-to-treat population consisted of all enrolled patients except three who were lost at follow-up. The study outcomes were 180-day mortality, EuroQoL (EQ)-5D-5L index values, and EQ-visual analog scale (EQ-VAS). In the modified intention-to-treat analysis, non-survivors were assigned a value of 0 for EQ-5D-5L and EQ-VAS.
Results
Within 180 days, 63/159 patients (39.6%) died in the helmet noninvasive ventilation group compared to 65/158 patients (41.1%) in the usual respiratory support group (risk difference − 1.5% (95% confidence interval [CI] − 12.3, 9.3, p = 0.78). In the modified intention-to-treat analysis, patients in the helmet noninvasive ventilation and the usual respiratory support groups did not differ in EQ-5D-5L index values (median 0.68 [IQR 0.00, 1.00], compared to 0.67 [IQR 0.00, 1.00], median difference 0.00 [95% CI − 0.32, 0.32; p = 0.91]) or EQ-VAS scores (median 70 [IQR 0, 93], compared to 70 [IQR 0, 90], median difference 0.00 (95% CI − 31.92, 31.92; p = 0.55).
Conclusions
Helmet noninvasive ventilation did not reduce 180-day mortality or improve HRQoL compared to usual respiratory support among patients with acute hypoxemic respiratory failure due to COVID-19 pneumonia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Compared with usual respiratory support, helmet noninvasive ventilation did not reduce 180-day mortality or improve health-related quality of life (HRQoL) in critically ill patients with acute hypoxemic respiratory failure due to coronavirus disease 2019. Invasive mechanical ventilation was an independent predictor of lower HRQoL. |
Introduction
The impact of acute hypoxemic respiratory failure due to coronavirus disease 2019 (COVID-19) extends beyond its short-term clinical outcomes, such as in-hospital morbidity and mortality. Survivors may continue to have poor health-related quality of life (HRQoL) for months after discharge [1,2,3]. Studies have demonstrated that patients with acute hypoxemic respiratory failure who were managed with invasive mechanical ventilation had decreased HRQoL [4,5,6]. However, the effect of noninvasive respiratory support on HRQoL is not well studied. This is particularly relevant as noninvasive respiratory support, including noninvasive ventilation and high-flow nasal oxygen, has increasingly been used during the COVID-19 pandemic, with the premise of preventing intubation and its sequalae. Noninvasive ventilation delivered through a helmet interface has been used in patients with acute hypoxemic respiratory failure due to COVID-19 to deliver prolonged uninterrupted treatments with high positive airway pressure to reduce self-inflicted lung injury [7, 8]. However, data on the effect of helmet noninvasive ventilation are limited to short-term mortality, and the effects on long-term mortality and quality of life in this population are unclear.
The Helmet-COVID trial evaluated whether helmet noninvasive ventilation compared with usual respiratory support would reduce 28-day all-cause mortality in patients with acute hypoxemic respiratory failure due to COVID-19 [9,10,11] and demonstrated that helmet noninvasive ventilation did not significantly reduce 28-day mortality [11]. In this report, we present the 180-day mortality and HRQoL results, which were pre-specified secondary outcome measures of the Helmet-COVID trial. We hypothesized that helmet noninvasive ventilation would reduce 180-day mortality and better HRQoL.
Methods
Study settings and populations
The Helmet-COVID trial was an investigator-initiated, pragmatic, multicenter randomized controlled trial conducted between 8 February 2021 and 16 November 2021 across seven sites in Saudi Arabia and 1 in Kuwait (Table S1) [9,10,11]. A priori or deferred written or witnessed verbal consent was obtained from all patients or surrogates per local approvals. The trial protocol was approved by the Institutional Review Boards in all participating sites.
This trial enrolled adult patients admitted to the intensive care unit (ICU) with acute hypoxemic respiratory failure (the ratio of arterial oxygen partial pressure to fraction of inspired oxygen (PaO2/FiO2) < 200 despite supplemental oxygen with at a flow rate ≥ 10 L/min) and suspected or confirmed COVID-19 pneumonia by reverse transcription-polymerase chain reaction. Exclusion criteria included prior intubation in the current hospital admission, cardiopulmonary arrest, Glasgow coma scale (GCS) of < 12, tracheostomy, upper airway obstruction, the requirement of > 1 vasopressor to maintain mean arterial pressure of > 65 mmHg, imminent intubation, do-not-intubate order, chronic carbon dioxide retention (PaCO2 > 45 mmHg), previous enrollment in this trial and heart failure as the primary cause of respiratory failure.
Eligible patients were randomly allocated to receive helmet noninvasive ventilation or usual respiratory support. In the helmet noninvasive ventilation group, pressure support was applied through a helmet (Subsalve, Middletown, Rhode Island) according to a written protocol, with initial settings of pressure support of 8–10 cmH2O, positive end-expiratory pressure (PEEP) of 10 cmH2O with FiO2 of 1, a flow rate of ≥ 50 L/min, an inspiratory rise time of 50 ms and end flow/cycling off of 50% of maximal inspiratory flow [9,10,11]. Interruptions of the helmet were avoided or kept at a minimum at least in the first 48 h [9,10,11]. Dexmedetomidine, but not other intravenous sedatives or narcotics, was allowed to improve comfort. If a patient continued to be intolerant to the helmet, the patient was managed according to the usual respiratory support. In the usual respiratory support group, patients were managed according to the clinical practices of each site, using mask noninvasive ventilation, high-flow nasal oxygen and standard oxygen [9,10,11].
The results of this analysis are reported per the CONSORT Statement PRO Extension (Consolidated Standards of Reporting Trials, Patient-Reported Outcomes) [12]. The modified intention-to-treat population consisted of all enrolled patients in the Helmet-COVID trial, except for three who were lost to follow-up for the 180-day outcomes. The HRQoL population consisted of patients who were alive at day 180 and had a response to the HRQoL interview. The per-protocol population consisted of all randomized patients who received the allocated intervention (helmet noninvasive ventilation for ≥ 1 h in the helmet noninvasive ventilation group, and no helmet noninvasive ventilation in the usual respiratory support group).
Data and study outcomes
Collected data are described in the Online Supplementary Methods. The pre-specified follow-up outcomes were all-cause 180-day mortality and HRQoL using EQ-5D-5L index values and EQ-VAS [13, 14]. The EQ-5D-5L questionnaire has five dimensions (mobility, self-care, usual activities, pain or discomfort, and anxiety/depression) with five levels of severity (no problems, slight problems, moderate problems, severe problems, extreme problems); thus describing 3125 possible health states [13]. Using a scoring algorithm based on public preferences, the EQ- 5D-5L index value is calculated and ranges from 1 (perfect health) to values below zero (health states valued worse than death with zero defined as a state equivalent to death) [14, 15]. Although the EQ-5D-5L instrument has been used in several patient populations in Saudi Arabia, there are no validated value sets that can be applied to critically ill patients [16]. Therefore, we calculated the index values based on the United States EQ-5D-5L value set [17]. We conducted sensitivity analyses of EQ-5D-5L index values using France and Japan set values in the modified intention-to-treat population. EQ-VAS provides a single global rating of self-perceived health and has values that range from 0 (worst imaginable health) to 100 (best imaginable health) [13].
Procedure
Data regarding vital status at day 180 were obtained from medical records and contact with patients or relatives by phone. Shortly after day 180, surviving patients were interviewed over the phone by a trained unblinded research coordinator using the EQ-5D-5L questionnaire as soon as possible. The patients were asked to describe the status of each dimension that best described their health on that day. If a patient could not provide answers, the next of kin was interviewed on behalf of the patient. Interviewers could make several attempts following day 180 to establish contact.
Statistical analysis
Continuous data were expressed as means with standard deviations or medians with interquartile ranges (IQR), according to normality distribution. Categorical data were expressed as numbers and percentages. We did not impute for missing values and did not correct for multiple testing. Post hoc sample size calculation is provided in the Online Supplementary Methods. A p < 0.05 was considered statistically significant, and analyses were conducted using SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
We reported baseline characteristics, interventions and co-interventions in the two groups among non-survivors and survivors by day 180 (HRQoL population).
In the modified intention-to-treat population, we compared 180-day mortality between the two groups using Chi-square test and reported the results as risk difference with 95% confidence interval (CI). We compared the time-to-death distributions between the two groups using Kaplan–Meier curves and log-rank tests.
In the modified intention-to-treat population, HRQoL population and per-protocol population, we used Mann–Whitney U test to compare the index values of EQ-5D-5L and EQ-VAS at 180 days of enrollment between the two groups. Quantile regression was used to calculate the median difference and 95% CI. In the HRQoL population, we used chi-square test or the Freeman-Halton extension of Fisher’s exact test to compare the distributions of individual dimension levels of EQ-5D-5L between the two groups.
We conducted a post hoc analysis comparison between intubated and non-intubated patients. We conducted a post hoc multivariable analysis evaluating the predictors of EQ-5D-5L and EQ-VAS at 180 days in the HRQoL population with the following covariates: receipt of helmet noninvasive ventilation, sex, acute physiology and chronic health evaluation (APACHE) II score, intubation, age, PaO2/FiO2 ratio at baseline and receipt of dexmedetomidine. Because both intubation and dexmedetomidine were post-randomization variables, we conducted a similar analysis restricted to day 28-survivors in the modified intention-to-treat population.
Results
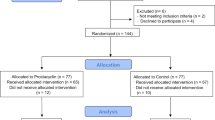
Of the 320 patients enrolled in the Helmet-COVID trial, the modified intention-to-treat population consisted of 317 patients with available 180-day outcomes (3 patients were lost to follow-up and had no data for mortality and HRQoL), and the HRQoL population consisted of 189 patients who survived to day 180 and all had HRQoL data (Online Supplement, Fig. S1 and Table S2). Patients in the HRQoL population were interviewed for EQ-5D-5L and EQ-VAS at a median of 186 days (IQR 182, 207.5) after randomization in the helmet noninvasive ventilation group and 186 days (IQR 181, 206) in the usual respiratory support group. The HRQoL questionnaire was answered by relatives/surrogate decision makers for 47/96 (49%) patients in the helmet noninvasive ventilation group and 46/93 (49.5%) in the usual respiratory support group.
Baseline characteristics
Among non-survivors by day 180, the baseline characteristics were balanced between the two groups, including age [median 64 years (IQR 53, 71) compared with 64 years (IQR 55, 72)], the prevalence of comorbidities (46/63 patients [70%] compared with 52/65 patients [80%]) and PaO2/FiO2 on enrollment (70 mmHg [IQR 58, 80] compared with 68.8 [55, 96]) (Table 1).
Similarly, among patients who were alive by day 180 and who were interviewed for HRQoL, the baseline characteristics were balanced between the two group, including age [median 54 years (IQR 43–62) compared with 56 years (IQR 49, 63)], the prevalence of comorbidities (62/96 patients [64.6%] compared with 63/93 patients [67.7%]) and PaO2/FiO2 on enrollment (77.1 [IQR 60.5, 98.6] compared with 88.8 [64.3, 114.3]) (Table 1).
Interventions and co-interventions
Among non-survivors by day 180, helmet noninvasive ventilation was used in 63/63 patients (100%) in the helmet noninvasive ventilation group and 2/65 patients (3.1%) in the usual respiratory support group. Co-interventions, including vasopressors, renal replacement therapy, corticosteroids and tocilizumab, were not different between the two groups. Dexmedetomidine infusion during noninvasive respiratory support was used in 36/63 patients (57.1%) in the helmet noninvasive respiratory support group and 22/65 patients (33.8%) in the usual respiratory group (Table 2). Other co-interventions are described in Table S3, Online Supplement.
Among patients who were alive by day 180 and were interviewed for HRQoL, helmet noninvasive ventilation was used in 89/96 patients (92.7%) in the helmet noninvasive ventilation group and in 2/93 patients (2.2%) in the usual respiratory support group. Co-interventions, including vasopressors, renal replacement therapy, corticosteroids and tocilizumab, were not different between the two groups. Dexmedetomidine infusion during noninvasive respiratory support was used in 33/96 patients (34.4%) in the helmet noninvasive ventilation group and 18/93 patients (19.4%) in the usual respiratory group (Table 2). Other co-interventions are described in Table S3, Online Supplement.
Modified intention-to-treat analysis
180-day mortality
In the modified intention-to-treat population, 63/159 patients (39.6%) died within 180 days in the helmet noninvasive ventilation group compared with 65/158 patients (41.1%) in the usual respiratory support group (risk difference − 1.5%, 95% CI − 12.3, 9.3; relative risk 0.96; 95% CI 0.74, 1.26, p = 0.78). Kaplan–Meier curves for mortality showed no difference in time-to-death distribution between the two groups (log-rank p = 0.86, Fig. 1).
Health-related quality of life
In the modified intention-to-treat cohort, the EQ-5D-5L index value was not different between the helmet noninvasive ventilation and the usual respiratory support groups (median 0.68 [IQR 0.00, 1.00], compared with 0.67 [IQR 0.00, 1.00], median difference 0.00 [95% CI − 0.32, 0.32; p = 0.91]) (Table 3 and Fig. 2A). The distributions of individual EQ-5D-5L dimension levels were statistically not different between the two groups (Fig. 2C). EQ-VAS value was not different between patients in the helmet noninvasive ventilation and the usual respiratory support groups (median 70 [IQR 0, 93], compared with 70 [0, 90], median difference 0 [95% CI − 31.92, 31.92; p = 0.55]) (Table 3 and Fig. 2B). Analysis of the per-protocol population and sensitivity analyses using France and Japan set values in the modified intention-to-treat population yielded consistent results (Tables S4 and S5, Online Supplement).
A, B and C Comparisons of EQ-5D-5L index values, EQ-VAS and EQ-5D-5L dimension levels between patients in the helmet noninvasive ventilation and usual respiratory support groups; none of the comparisons were statistically significantly different between the two groups. A and B Distribution of the EQ-5D-5L index values and EQ-VAS as horizontally stacked proportions in patients assigned to helmet noninvasive ventilation and usual respiratory support in the modified intention-to-treat population. C Distributions of individual EQ-5D-5L dimension levels in the HRQoL population in patients assigned to helmet noninvasive ventilation and usual respiratory support (see also Table S6, Online Supplement). D, E and F Comparisons of EQ-5D-5L index values, EQ-VAS and EQ-5D-5L dimension levels between patients who were intubated and those who were not intubated; all comparisons were statistically significantly different between the two groups. D and E Distribution of the EQ-5D-5L index values and EQ-VAS as horizontally stacked proportions in patients who were intubated and those who were not intubated in the modified intention-to-treat population. F Distributions of individual EQ-5D-5L dimension levels in patients who were intubated and those who were not in the HRQoL population (see also Tables S8 and S9, Online Supplement)
Analysis of the HRQoL population
In the HRQoL population, the EQ-5D-5L index values were similar between the helmet noninvasive ventilation and usual respiratory support groups (median 0.94 [IQR 0.72, 1.00], compared with 1 [IQR 0.72, 1.00], median difference 0.00 [95% CI − 0.09, 0.09; p = 0.91]) (Table 3). Of the patients assigned to helmet noninvasive ventilation and usual respiratory support and were alive by day 180, 39/96 (40.6%) and 35/93 (37.6%) reported problems in mobility, 29/96 (30.2%) and 29/93 (31.2%) reported problems in self-care, 34/96 (35.4%) and 35/93 (37.6%) reported problems in usual activities, 37/96 (38.5%) and 37/93 (39.8%) reported pain or discomfort, 28/96 (29.2%) and 27/93 (29%) reported anxiety or depression, respectively. None of the comparisons were statistically different between the two groups (Table S6, Online Supplement).
In the HRQoL population, the median EQ-VAS for patients who received helmet noninvasive ventilation versus usual respiratory support was similar (median 90, IQR 75–100 versus 85, IQR 75–99, respectively; median difference 5, 95% CI − 1.33, 11.33; p = 0.46, Table 3). In the 180-day survivors, the index values were similar between the helmet noninvasive ventilation and usual respiratory support groups (median 0.94, IQR 0.72, 1, versus 1, IQR 0.72, 1, median difference 0, 95% CI − 0.09, 0.99, 11.33; p = 0.91). Analysis in the per-protocol population and sensitivity analyses using France and Japan set values yielded consistent results (Tables S4 and S5, Online Supplement).
In the HRQoL population, the multivariable analysis demonstrated that helmet noninvasive ventilation compared with usual respiratory support was not associated with a difference in EQ-5D-5L index values (median difference 0.0, 95% CI − 0.06, 0.06; p > 0.99), while intubation was independently associated with reduced EQ-5D-5L index values (median difference − 0.21, 95% CI − 0.26, − 0.15; p < 0.0001). Helmet noninvasive ventilation compared with usual respiratory support was not associated with a difference in EQ-VAS (median difference 0.5, 95% CI − 3.9, 4.9; p = 0.82, while intubation (median difference − 11.68, 95% CI − 16.41, − 6.92; p < 0.0001) and the use of dexmedetomidine (median difference − 7.88, 95% CI − 13.33, − 2.34; p = 0.006) were independently associated with reduced EQ-VAS. Multivariable analysis including 28-day survivors in the modified intention-to-treat analysis demonstrated similar results (Table S7, Online Supplement).
Post hoc analysis of intubated versus non-intubated patients
Additional post hoc analyses comparing intubated and non-intubated patients in the modified intention-to-treat and HRQoL populations are provided in Table S8. The EQ-5D-5L index value and EQ-VAS were significantly lower in intubated compared with non-intubated patients in the modified intention-to-treat population (median 0, IQR 0, 0.42, versus 1, IQR 0.72, 1; p < 0.0001, and 0, IQR 0, 50 versus 90, IQR 75, 100; p < 0.0001), and HRQoL population (median 0.70, IQR 0.64, 0.85, versus 1, IQR 0.81, 1; p < 0.0001, and median 80, IQR 60, 85 versus 90, IQR 80, 100; p < 0.0001; Fig. 2D, E and Table S8). Distributions of individual EQ-5D-5L dimension levels among the HRQoL population in intubated and non-intubated patients showed that patients who were intubated patients had lower levels for all dimensions (p < 0.0001, Table S9).
Discussion
In this multicenter randomized clinical trial of patients with acute hypoxemic respiratory failure due to COVID-19, we observed no statistically significant differences in mortality or HRQoL at 180 days among patients assigned to the helmet noninvasive ventilation versus usual respiratory support. The results are consistent with the study outcomes observed at the 28-day follow-up.
Several studies have examined HRQoL after severe COVID-19, and the results differed according to the studied populations. The EQ‑5D‑5L index values and EQ-VAS in our study are comparable to those observed in COVID STEROID 2 trial (dexamethasone 12 mg versus 6 mg in patients with COVID-19). Among the 6-month survivors of the COVID STEROID 2 trial (n = 574, median age 61 years), median EQ‑5D‑5L index values were 0.93 (IQR 0.81, 1) and 0.92 (IQR 0.77, 1), respectively, and EQ-VAS scores were 80 (IQR 65, 95) and 80 (IQR 65, 90), respectively [18]. Our study reports HRQoL that are higher than what has been reported in observational studies, probably reflecting patient selection. For example, patients were enrolled in our trial if they were able to follow instructions and excluded if they had a Glasgow coma scale < 12, tracheostomy, do-not-intubate orders or chronic carbon dioxide retention. These eligibility criteria would lead to the selection of a healthier and generally younger population than observed in observational studies, which usually have limited selection criteria. For example, a multicenter prospective study in Spain of survivors from COVID-19 ARDS (n = 91, mean age 65.5 years) found that the EQ-VAS at 180 days was 66.4 ± 18.3 and EQ-5D-5L index value 0.71 ± 0.25 [4]. The prospective multicenter post-hospitalization COVID-19 (PHOSP-COVID) cohort study (2320 participants from the United Kingdom, mean age 58.7 years) found that median EQ-5D-5L index value at 5 months to be 0.74 (IQR 0.64, 0.88) [5]. A prospective study from Italy (n = 205, age 64.5 years) found that COVID-19 critically ill survivors had reduced EQ-5D-5L index values and EQ-VAS score at 6 months [6].
The effect of noninvasive respiratory support for the acute management of respiratory failure on HRQoL is not well studied. A systematic review included two studies that evaluated HRQoL in 134 patients with do-not-intubate status who received noninvasive ventilation for acute respiratory failure and found that HRQoL was not reduced compared with baseline in survivors [19]. A single-center randomized clinical trial in patients with non-COVID-19 ARDS compared helmet versus mask noninvasive ventilation [20]. One-year mortality was lower in the helmet group [20]. At 1 year, patients in the helmet group were more likely to be functionally independent [20].
On the other hand, our study showed that HRQoL was significantly lower in patients who received invasive mechanical ventilation. Similar findings were seen in other studies [4,5,6]. The PHOSP-COVID study found that patients who received invasive mechanical ventilation were less likely to report full recovery at one year (odds ratio 0.42, 95% CI 0.23, 0.76) [5]. A prospective study at 16 Italian ICUs of COVID-19 critically ill survivors who received mechanical ventilation found that the duration of invasive mechanical ventilation was among the significant determinants of HRQoL assessed by the 15D instrument [6].
Strengths of this study include being a pre-planned follow-up analysis of a multicenter trial with a limited number of loss to follow-up. The study assessed HRQoL by generic scales (EQ-5D-5L and EQ-VAS) that has been used to evaluate survivors of acute respiratory failure [21], and critically ill patients in general [4, 5, 18]. These factors increase the results' internal and external validity. Limitations include having no baseline HRQoL data, the inability of the EQ-5D-5L instrument to cover all aspects of HRQoL that may be important to patients, and the lack of EQ-5D-5L value sets for Saudi Arabia and Kuwait. However, analyses using set values of three different countries (United States, France and Japan) showed consistently no difference between the two study groups. Assessment of HRQoL was performed by unblinded coordinators. Dexmedetomidine was used more frequently in the helmet noninvasive ventilation group; but the effect of dexmedetomidine on outcomes of patients managed with noninvasive ventilation remains uncertain. Given the condition of patients, almost half of the responses for HRQoL, equally in both groups, were obtained from surrogate decision makers. This might have affected the measurement of HRQoL, although unlikely to have introduced a bias in the in-between group difference. In this pragmatic trial, we had no data on patients' effort by physiological techniques (e.g. esophageal pressure) to determine if there were differences in inspiratory efforts between the two groups.
Conclusions
Compared with usual respiratory support, helmet noninvasive ventilation did not reduce 180-day mortality or improve HRQoL in patients with acute hypoxemic respiratory failure due to COVID-19. Invasive mechanical ventilation was an independent predictor of lower HRQoL.
Data availability
Data is available from the corresponding author upon reasonable request as per the regulations of King Abdullah International Medical Research Center (KAIMRC) within 3 years of the publication.
References
Bryson WJ (2021) Long-term health-related quality of life concerns related to the COVID-19 pandemic: a call to action. Qual Life Res 30:643–645
Crook H, Raza S, Nowell J, Young M, Edison P (2021) Long COVID-mechanisms, risk factors, and management. BMJ (Clin Res Ed) 374:n1648
Santus P, Tursi F, Croce G, Di Simone C, Frassanito F, Gaboardi P, Airoldi A, Pecis M, Negretto G, Radovanovic D (2020) Changes in quality of life and dyspnoea after hospitalization in COVID-19 patients discharged at home. Multidiscip Respir Med 15:713
Taboada M, Moreno E, Cariñena A, Rey T, Pita-Romero R, Leal S, Sanduende Y, Rodríguez A, Nieto C, Vilas E (2021) Quality of life, functional status, and persistent symptoms after intensive care of COVID-19 patients. Br J Anaesth 126:e110–e113
Group P-CC, Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: a prospective observational study. Lancet Respir Med S2213–2600 (2222) 00127–00128
Gamberini L, Mazzoli CA, Sintonen H, Colombo D, Scaramuzzo G, Allegri D, Tonetti T, Zani G, Capozzi C, Giampalma E (2021) Quality of life of COVID-19 critically ill survivors after ICU discharge: 90 days follow-up. Qual Life Res 30:2805–2817
Grieco DL, Maggiore SM, Roca O, Spinelli E, Patel BK, Thille AW, Barbas CSV, de Acilu MG, Cutuli SL, Bongiovanni F, Amato M, Frat JP, Mauri T, Kress JP, Mancebo J, Antonelli M (2021) Non-invasive ventilatory support and high-flow nasal oxygen as first-line treatment of acute hypoxemic respiratory failure and ARDS. Intensive Care Med 47:851–866
Menga LS, Delle Cese L, Rosa T, Cesarano M, Scarascia R, Michi T, Biasucci DG, Ruggiero E, dell’Anna AM, Cutuli SL, Tanzarella ES, Pintaudi G, De Pascale G, Sandroni C, Maggiore SM, Grieco DL, Antonelli M (2022) Respective effects of helmet pressure support, continuous positive airway pressure and nasal high-flow in hypoxemic respiratory failure: a randomized crossover clinical trial. Am J Respir Crit Care Med. https://doi.org/10.1164/rccm.202204-0629OC
Arabi YM, Tlayjeh H, Aldekhyl S, Al-Dorzi HM, Abdukahil SA, Al Harbi MK, Al Haji H, Al Mutairi M, Al Zumai O, Al Qasim E, Al Wehaibi W, Al Qahtani S, Al-Hameed F, Chalabi J, Alshahrani M, Albrahim T, Alharthy A, Mady A, Bin Eshaq A, Al Bshabshe AA, Al Aseri Z, Al Duhailib Z, Kharaba A, Alqahtani R, Al Ghamdi A, Altalag A, Alghamdi K, Almaani M, Algethamy H, Al Aqeily A, Al Baseet F, Al Samannoudi H, Al Obaidi M, Ismaiel YT, Al-Fares AA (2021) Helmet non-invasive ventilation for COVID-19 patients (helmet-COVID): study protocol for a multicentre randomised controlled trial. BMJ Open 11:e052169
Arabi Y, Aldekhyl S, Al Qahtani S, Al-Dorzi HM, Abdukahil SA, Jose J, Al Harbi MK, Al Haji H, Al Mutairi M, Al Zumai O, Al Qasim E, Al Wehaibi W, Alshahrani M, Albrahim T, Mady A, Al Bshabshe A, Al Aseri Z, Al Duhailib Z, Kharaba A, Alqahtani R, Algethamy H, Alfaris O, Alnafel O, Al-Fares AA, Tlayjeh H (2022) Helmet noninvasive ventilation for COVID-19 patients (helmet-COVID): statistical analysis plan for a randomized controlled trial. Trials 23:105
Arabi YM, Aldekhyl S, Al Qahtani S, Al-Dorzi HM, Abdukahil SA, Al Harbi MK, Al Qasim E, Kharaba A, Albrahim T, Alshahrani MS, Al-Fares AA, Al Bshabshe A, Mady A, Al Duhailib Z, Algethamy H, Jose J, Al Mutairi M, Al Zumai O, Al Haji H, Alaqeily A, Al Aseri Z, Al-Omari A, Al-Dawood A, Tlayjeh H, Saudi Critical Care Trials G (2022) Effect of helmet noninvasive ventilation vs usual respiratory support on mortality among patients with acute hypoxemic respiratory failure due to COVID-19: the HELMET-COVID randomized clinical trial. JAMA 328:1063–1072
Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD, Group CP (2013) Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA 309:814–822
Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, Bonsel G, Badia X (2011) Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 20:1727–1736
Van Hout B, Janssen M, Feng Y-S, Kohlmann T, Busschbach J, Golicki D, Lloyd A, Scalone L, Kind P, Pickard AS (2012) Interim scoring for the EQ-5D-5L: map** the EQ-5D-5L to EQ-5D-3L value sets. Value Health 15:708–715
Ramos-Goñi JM, Pinto-Prades JL, Oppe M, Cabasés JM, Serrano-Aguilar P, Rivero-Arias O (2017) Valuation and modeling of EQ-5D-5L health states using a hybrid approach. Med Care 55:e51
Althemery A (2021) Application of the EQ-5D in the middle east: a systematic review focusing on patients living in the Kingdom of Saudi Arabia. J Multidiscip Healthc 14:1101–1106
van Hout BA, Shaw JW (2021) Map** EQ-5D-3L to EQ-5D-5L. Value Health 24:1285–1293
Granholm A, Kjær M-BN, Munch MW, Myatra SN, Vijayaraghavan BKT, Cronhjort M, Wahlin RR, Jakob SM, Cioccari L, Vesterlund GK, Meyhoff TS, Helleberg M, Møller MH, Benfield T, Venkatesh B, Hammond NE, Micallef S, Bassi A, John O, Jha V, Kristiansen KT, Ulrik CS, Jørgensen VL, Smitt M, Bestle MH, Andreasen AS, Poulsen LM, Rasmussen BS, Brøchner AC, Strøm T, Møller A, Khan MS, Padmanaban A, Divatia JV, Saseedharan S, Borawake K, Kapadia F, Dixit S, Chawla R, Shukla U, Amin P, Chew MS, Wamberg CA, Bose N, Shah MS, Darfelt IS, Gluud C, Lange T, Perner A (2022) Long-term outcomes of dexamethasone 12 mg versus 6 mg in patients with COVID-19 and severe hypoxaemia. Intensive Care Med 48:580–589
Wilson M, Majzoub A, Dobler C, Curtis J, Nayfeh T, Thorsteinsdottir B, Barwise A, Tilburt J, Gajic O, Montori V (2018) Noninvasive ventilation in patients with do-not-intubate and comfort-measures-only orders: a systematic review and meta-analysis. Crit Care Med 46:1209–1216
Patel BK, Wolfe KS, MacKenzie E, Salem D, Esbrook CL, Pawlik AS, Stulberg M, Kemple C, Teele M, Zeleny E (2018) One year outcomes in patients with acute respiratory distress syndrome enrolled in a randomized clinical trial of helmet versus facemask noninvasive ventilation. Crit Care Med 46:1078
Needham DM, Sepulveda KA, Dinglas VD, Chessare CM, Friedman LA, Bingham CO III, Turnbull AE (2017) Core outcome measures for clinical research in acute respiratory failure survivors. An international modified Delphi consensus study. Am J Respir Crit Care Med 196:1122–1130
Acknowledgements
The authors would like to thank all the participating patients and their families, as well as the members of the Data and Safety Monitoring Board (DSMB): DSMB Chair: Nicholas S. Hill, MD (Professor of Medicine, Chief of Pulmonary, Critical Care and Sleep Division, Tufts Medical Center, Boston, Massachusetts, USA), DSMB members: Stefano Nava, MD (Professor of Respiratory Medicine University of Bologna, Chief of the Respiratory and Critical Care, Alma Mater Studiorum University of Bologna. IRCCS Sant’Orsola Hospital, Respiratory and Critical Care Unit University of Bologna, Italy), James Mojica, MD (Vice Chief & Clinical Director of the Division of Pulmonary & Critical Care Medicine; Massachusetts General Hospital, USA) and Michael Harhay, PhD (Assistant Professor of Epidemiology and Medicine-Pulmonary and Critical Care, Department of Biostatistics, Epidemiology and Informatics, University of Pennsylvania, USA).
Saudi Critical Care Trials Group includes: Management Committee: Yaseen M. Arabi, Hasan M. Al-Dorzi, Haytham Tlayjeh, Sara Aldekhyl, Saad Al-Qahtani, Mohammed Khulaif Al-Harbi, Mohammad Al-Mutairi, Hussain Al-Haji, Omar Al-Zumai, Ahmed Alaqeily, Sheryl Ann Abdukahil, Eman Al-Qasim, Jesna Jose. Writing Committee: Yaseen M. Arabi, Hasan Al-Dorzi, Sheryl Ann Abdukahil. Data Safety & Monitoring Board: Nicholas Hill, Stefano Nava, James Mojica, Michael Harhay. Collaborators: Al Amiri Hospital, Kuwait: Abdulrahman Al-Fares, Ahmed Almumin, James Albert, Israr Khan, Muhammad Ayaz. Aseer Central Hospital, Abha: Ali Al Bshabshe, Munir Mustafa Aldammad, Nasser M. Alwadai, Om Prakash Palanivel. King Abdulaziz Medical City, Riyadh: Yaseen M. Arabi, Hasan M. Al-Dorzi, Haytham Tlayjeh, Mohammad Al Harbi, Sara Aldekhyl, Saad Al Qahtani, Abdulaziz Al-Dawood, Sheryl Ann I. Abdukahil, Eman Al Qasim, Jesna Jose, Wedyan Al Wehaibi, Musharaf Sadat, Lara Afesh, Felwa Bin Humaid, Mohammad Al Mutairi, Hussain Al Haji, Omar Al Zumai, Ahmed Alaqeily, Yassin Ismaiel, Faisal Al Baseet, Mohammad Al Obaidi, Edgardo Tabhan, Rami Al Khalid, Omar Al Fares, Abdullah Al Suayb, Hashem Sammanoudi, Victoria Burrows, Amal Matroud, Brintha Naidu. King Abdulaziz University Hospital, Jeddah: Haifa Algethamy, Sheryl Lungue, Liyakat Khan, Moataz Jaber, Saleh Baaziz, Shehla Nuzhat. King Fahd Hospital of the University, Imam Abdulrahman Bin Faisal University, Dammam: Mohammed S Alshahrani, Talal Ali Albrahim, Laila Perlas Asonto, Charlene Mapusao, Arivukodhi Muthu, Abdulaziz Saad AlGhamdi, Carmelo Angala. King Fahad Hospital, Madinah: Ayman Kharaba, Mohamed Hussien, Ahmad AlFar, Salman Al Asiri, Anas Al Solami. King Faisal Specialist Hospital & Research Center, Riyadh: Zainab Al Duhailib, Mahmoud Abu Riash, Haya Al Othaimeen, Rozeena Huma. King Saud Medical City, Riyadh: Ahmed Mady, Naif Abdulrahman Aldosari, Khalid Abdullah Alreyes, Arul Prasath Lakshmanan, Alzahra Al Obaed, Mobarak Almushhen, Fhausia Hali, Ika Fibriantini, Bobby Rose Marasigan, Katrina Baguisa, Saleh Ali Almahwi. King Abdulaziz Hospital, Al Ahsa: Jamal Chalabi. King Abdulaziz Medical City, Jeddah: Fahad Al-Hameed. King Khalid Hospital, Najran: Abdulhadi Bin Eshaq. King Khalid University Hospital, Riyadh: Rakan Alqahtani. King Salman Specialist Hospital, Hail: Omar Alnafel. Saudi Critical Care Society: Zohair Al Aseri, Awad Al-Omari.
Funding
The study was funded by King Abdullah International Medical Research Center (Grant RC20/306/R), Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Consortia
Contributions
YA administrative, technical, or logistic support, analysis and interpretation of the data, collection and assembly of data, conception and design, critical revision for important intellectual content, drafting of the article, final approval of the article, obtaining of funding, provision of study materials or patients and statistical expertise. SD, SAQ, EAQ, MAH, AK, TI, MS, AF, AAB, AM, ZAD, HG, MAM, OAZ, HAH, AA, WAW, ZAA, AO, HT, AD administrative, technical, or logistic support, collection and assembly of data, final approval of the article and provision of study materials or patient. HD, SA, EAQ, administrative, technical, or logistic support, analysis and interpretation of the data, collection and assembly of data, critical revision for important intellectual content, drafting of the article, final approval of the article and provision of study materials or patient. JJ analysis and interpretation of the data, critical revision for important intellectual content, final approval of the article and statistical expertise.
Corresponding author
Ethics declarations
Conflicts of interest
HMA-D reported receiving honoraria for educational activities from Sanofi outside the submitted work. No other disclosures were reported.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The members of the Saudi Critical Care Trials Group are listed in the Acknowledgement section of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arabi, Y.M., Al-Dorzi, H.M., Aldekhyl, S. et al. Long-term outcomes of patients with COVID-19 treated with helmet noninvasive ventilation or usual respiratory support: follow-up study of the Helmet-COVID randomized clinical trial. Intensive Care Med 49, 302–312 (2023). https://doi.org/10.1007/s00134-023-06981-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-023-06981-5