Abstract
Headwaters in alpine regions and their biodiversity are particularly threatened by climatic changes. Most predictions on their response to climate change are based on modeling approaches. Empirically gained data rarely exist for glacially influenced and groundwater-fed headwaters. In 2019, long-term monitoring was initiated at 15 springs, 8 springbrooks and 2 brooks in the UNESCO Biosphere Reserve Engiadina Val Müstair. The goal was to gain data on hydro-ecological aspects over several decades to understand whether (1) the environmental conditions change over time and (2) how these changes influence the composition of the species assemblages. Water temperature loggers were installed, pH, electrical conductivity, oxygen, nutrients and discharge were measured three times per year, and ecomorphological features were mapped two times per year. The meio- and macrofauna was sampled in 2019, 2020 and 2021 with a semi-quantitative approach. The results of the first 5 years of monitoring show that the physico-chemistry, water temperature and discharge confirm the stable character typical for groundwater-fed systems. Certain seasonal variability is evident, which possibly indicates an influence of permafrost or snow meltwater. The composition of the species assemblages differs significantly between sites but stays relatively constant over time within a site. Elevation and the availability of wood—parameters indicating forestation—significantly influence the species composition. This study provides a solid baseline on the environmental conditions and the fauna in springs and springbrooks in the Central Alps, which is needed for a proper interpretation of changes identified on a long-term basis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Freshwater worldwide are faced with significant alterations caused by global change. Small water bodies in particular are threatened by climate and land use changes, which lead, for example, to altered discharge regimes and high pesticide loads (Biggs et al. 2017; Hogan et al. 2023; Liess et al. 2023). Alpine running waters are of special concern as their discharge often depends on glacier meltwater, which, scenarios predict, will decrease significantly in the coming decades (Brighenti et al. 2019). The annual precipitation regime will change considerably, with less snow in winter and fewer rainfall events in summer, which will have a strong impact on the discharge regime (Michel et al. 2022). This could lead to an increase in water temperatures in Alpine rivers (Michel et al. 2022) and periodic drying of certain streams (Brighenti et al. 2019). The changing discharge regime as well as potentially increasing water temperatures could influence the composition of invertebrate assemblages in subalpine and alpine running waters (e.g., Küry et al. 2018). Long-term data analyses of rivers in several ecoregions in Europe demonstrate a shift in species composition in recent decades (Jourdan et al. 2018). Climatic changes are likely to lead to an expansion of the distribution range of generalists and a loss of specialists, e.g., cold-stenothermal species, in alpine freshwaters (Bässler et al. 2010; Timoner et al. 2020). This also appears to apply for species adapted to streams fed by glacier meltwater (e.g., Lencioni 2022).
Springs are usually relatively stable habitats fed by groundwater (e.g., van der Kamp 1995; Fattorini et al. 2016), with less variation of the water temperature and the discharge regime compared to springbrooks, i.e., the hypocrenal sensu Illies and Botosaneanu (1963), and brooks, i.e., the epirhithral sections of streams (Illies 1961). In the Alps, springs are additionally fed by glacier meltwater and permafrost subsurface runoff. This buffers the water temperature at a low but constant level and influences the discharge regime (von Fumetti et al. 2017). Due to their environmental stability, springs are partly inhabited by species adapted to this particular environment. Taxa with a high proportion of crenobiontic species, which are restricted to springs, i.e., the crenal, include Hydrachnidia, Trichoptera, and certain Diptera families such as the Stratiomyidae. The proportion of crenobiontic species in a spring seems to decrease with altitude, probably because of the constantly low water temperatures in high alpine springs (von Fumetti et al. 2017) and the reduced availability of habitat and food (Wigger et al. 2015). In the future, crenobiontic species could benefit from forest expansion (Wigger et al. 2015), while endemic alpine taxa, also but not exclusively found in springs, appear to be highly vulnerable to climatic changes (e.g., Niedrist and Füreder 2023).
Most studies investigating the potential impacts of climate change on freshwater species in running waters rely on species traits (Hering et al. 2009; Niedrist and Füreder 2023) or on space-time models and often focus on rivers with large catchments (Michel et al. 2022). Empirical approaches to analyzing the effects of climate change on the physico-chemical properties and the composition and function of species assemblages in running waters are still rare. Due to their supposed ecological and biological stability, springs and their springbrooks are ideal field laboratories for monitoring possible changes in abiotic conditions and species composition. To empirically record possible changes in species diversity in springs, springs and their springbrooks have to be studied over the long term. Environmental stability seems to enhance the stability of spring faunal assemblages (Gerecke et al. 2006). However, only a few demonstrate such stability over time. Long-term studies currently exist for a few springs in the Berchtesgaden National Park in Germany (e.g., Gerecke et al. 2009) and the Adamello-Brenta Nature Park in Italy (e.g., Cantonati et al. 2007), but they do not include springbrooks. A review on methodological aspects for long-term studies on springs is given by Cantonati et al. (2022).
Protected areas are ideal model regions for observing environmental changes due to their comparatively low anthropogenic pressures. This applies in particular to the UNESCO Engiadina Val Müstair Biosphere Reserve (UBEVM) with the Swiss National Park (SNP) as its core zone, which has been strictly protected as an IUCN 1a reserve since 1914. The first spring surveys were carried out in the 1930s by Adolf Nadig (Nadig 1942), followed by partly taxonomic investigations in the following decades (e.g., Aubert 1965; Bader 1975; Robinson et al. 2008; von Fumetti and Blattner 2017). In 2019, long-term monitoring of springs and springbrooks was initiated in the UBEVM. In this paper, we present the conceptualization of our research approach and report on initial results of the first 5 years. We hypothesized (1) that the environmental conditions (water temperature, discharge, and water chemistry) of the springs are stable and variability increases downstream and (2) that the faunistic assemblages are adapted to environmental stability and the species composition is not variable. With this initial study we set the baseline to better understand in the future how climate change is possibly altering environmental conditions and influence the biodiversity of alpine springs and brooks.
Materials and methods
Study site and study design
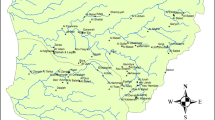
The UNESCO Engiadina Val Müstair Biosphere Reserve (UBEVM) is located in the Engadine in the eastern part of the canton of Grisons in Switzerland. With a total area of 449 km2, it is divided in three zones, the core zone (SNP) (170 km2), buffer zone (174 km2) and transition zone (105 km2). Compared to other inner Alpine regions, the Engadine has a relatively dry climate with the highest rainfall in summer (~ 100 mm in July and August) (Haller et al. 2013). Geologically, the region is very homogeneous and consists mainly of carbonate Engadine dolomite. At some sites, evaporites are present that enrich the water with sulfate (e.g., Raibler group) (Schlüchter et al. 2013). In some parts of the UBEVM a certain influence of permafrost is evident (Keller 2013). Outside the SNP, extensive livestock farming and hay meadows are common. The forested parts of the UBEVM are dominated by Scots pine (Pinus sylvestris), Swiss stone pine (Pinus cembra) and European larch (Larix decidua).
Fifteen springs, eight springbrooks and two larger brooks were selected for long-term monitoring (Table 1). The regions of Il Fuorn, Val Ftur, Buffalora and Spöl are located in the SNP. Las Multas and Alp Champatsch are located in Val Müstair, which is in the buffer zone and transition zone of the UBEVM. The Ravitschana and Tiatscha Sot sites in Val S-charl are situated in the transition zone of the UBEVM, whereas the Tamangur sites are in the buffer zone. In the buffer and transition zone of the UBEVM a certain anthropogenic influence cannot be excluded because of the presence of livestock. All springs and springbrooks were known to have a perennial discharge. Earlier data are available for some of the springs (e.g., Nadig 1942; von Fumetti and Felder 2014).
The altitude ranges from 1660 m a.s.l. (Punt Periv spring, SNP) to 2230 m a.s.l. (Q1_Bescha, Val Müstair). Most of the sites are situated in soft chaparral dominated by Scots pine, Swiss stone pine and European larch. Only Q1_Bescha is located above the tree line, whereas Q2_Bescha and the corresponding brook are situated in open land extensively frequented by livestock. The influence of livestock is also apparent at the springs Q2_Posa, Q_Taman and Q_Tiats. There is obviously no influence of livestock in the SNP, but the frequent presence of deer and chamois is evident at the springs Q_VF2, Q_VF3 and Punt Periv.
Sampling and measurements began at all springs in the SNP and Val S-charl in May 2019, and at all other sites in June 2020. The Tamangur springbrook was included in the monitoring in June 2021. The sampling campaigns took place in all years at the end of May (i.e., in spring), in mid July (i.e., in summer) and in the first half of October (i.e., in autumn). Physico-chemistry, ions and nutrients were measured three times per year until October 2023. Discharge was measured at least twice a year (spring and autumn) at most of the sites and the map** of the sites was done at all sites twice a year (spring and autumn) until October 2023. In 2022, all measurements were done only once (July). In accordance with Lubini et al. (2014), faunistic sampling was always performed between 8 and 15 July in 2019, 2020 and 2021. In 2022 and 2023, faunistic sampling was replaced by eDNA-metabarcoding, which has not yet been analyzed and will be not included in this initial analysis. The total number of samples and measurements per site is given in Online Resource 1.
Environmental parameters
Temperature and light loggers (Hobo Pendant® MX2202 Temp/light Logger, Onset, Bourne, USA) were placed at each site, ideally at the outflow or alternatively at a location where a constant overflow was ensured. Loggers were attached to a stone and secured using a large nail (~ 25 cm long). Where possible, a shady location was chosen to prevent the loggers from heating up because of solar radiation. The loggers were read out in spring and autumn. Monthly mean, minimum and maximum as well as amplitude were calculated for each month where data were available. Water temperatures < 0 °C were not considered as they indicate that a logger was temporarily dry in winter. However, exceptionally high values in summer were not omitted as measurements with the portable meter confirmed high water temperatures at some sites during this season. A certain amount of data loss due to depleted batteries or the loss of loggers, e.g., due to trampling by livestock, was inevitable at almost all sites (Online Resource 1). For seven sites (Q_VF2, Q_VF, Q_Tiats, Q_Ravit, Q1_Bescha, Qbr_Ravit and Laider) continuous temperature data were available from June 2020 until May 2023. These data were plotted, and significance of change over time was tested using the Mann-Kendall test. Plotting of the data and time series analysis was performed with PAST 4.13 (Hammer & Harper 2006).
Electrical conductivity (EC; µS/cm at 25 °C), pH value, oxygen content (mg/l) and saturation (%) in water were measured using a portable meter (WTW Multiline® Multi 3630 IDS, WTW). The measurements were taken alongside the temperature and light loggers. A water sample was taken to analyze the nutrient [ammonia (-NH4+), phosphate (-PO43−) and nitrate (-NO3−)] and ion content [natrium (-Na+), potassium (-K+), calcium (-Ca2+), magnesium (-Mg2+), sulfate (-SO42−), chloride (Cl−) and fluoride (-F−)]. The concentrations (all in mg/l) were measured with ICP-OES (SPECTRO MS, Spectro Analytical Instruments GmbH, Kleve, Germany) by the laboratory of Environmental Geosciences at the University of Basel.
Discharge was evaluated by measuring the cross section and flow velocity as accurately as possible at a well-defined cross section. The flow velocity was measured using a hydrometric vane with a micro vane for shallow water depths (Schiltknecht, Gossau, Switzerland). To account for measurement inaccuracies the calculated discharge was also converted into discharge classes according to Hoffsten and Malmqvist (2000): 1 (< 1 l/s); 2 (> 1—< 5 l/s); 3 (> 5—< 10 l/s); 4 (> 10—< 20 l/s); 5 (> 20 l/s).
The substrate composition and other ecomorphological parameters were documented based on the evaluation sheet of the Swiss Federal Office for the Environment (FOEN, Lubini et al. 2014). The substrate cover was mapped in five classes of areal coverage: 0 (0%); 1 (1–20%); 2 (20–40%); 3 (40–60%); 4: (60–90%), 5: (> 90%). An average value was calculated from all map** events resulting in a substrate cover per substrate and spring between 0 and 5. A certain variation in the visual assessment was evident but only between two classes, e.g., between class 1 (1–20% coverage) and 2 (20–40% coverage).
Faunistic sampling
For the faunistic sampling, a hand-net (mesh size: 200 µm) was used. The sampling design was based on the technique of the “Macrozoobenthos” module of the FOEN’s modular-stepwise procedure of the (Bundesamt für Umwelt 1998; 2019) and the recommendations of Cantonati et al. (2022). After map** the substrate composition, eight sub-samples were taken for each substrate present. The samples were taken in the uppermost 10 m of the spring or in a defined section of the springbrook. If fewer than eight substrates were present at a site, additional sub-samples were taken from the substrates that covered a large area according to the prior map**. The entire sample was pooled and then divided into a fine and a coarse fraction using a 200-µm hand net and a 1000-mm sieve. The fine fraction was preserved directly in 100% ethanol, whereas the coarse fraction was first separated from coarse inorganic substrates, i.e., gravel and stones, before preservation (Bundesamt für Umwelt 2019). Large specimens visible by eye were preserved directly in small vials.
The samples were sorted in the laboratory, and specimens were identified to the lowest possible taxonomic level, mostly genus or species level (e.g., Studemann et al. 1992; Amann et al. 1994; Waringer and Graf 2011; Lubini et al. 2012; Glöer 2017). Taxonomically challenging groups such as Chironomidae, Ceratopogonidae, Copepoda and Ostracoda as well as small instars were only identified to family level or higher. Not every indicator group proposed by Gerecke et al. (2011) was considered: Ostracoda, Copepoda, Chironomidae and diatoms are species rich taxa in springs (see, e.g., Gerecke and Franz 2006; Cantonati et al. 2007; Fattorini et al. 2016) but cannot be regularly identified [or not considered on a regular basis (diatoms)] in the UBEVM because of financial and time constraints.
Faunistic data analysis
For all springs except the springs in Val Müstair, a comparison among the years 2019, 2020 and 2021 was possible. Species richness (i.e., the number of taxa), abundance and Shannon diversity (H’) were calculated for each spring and each year. The percentage of taxa occurring each year was calculated as a measure of the stability of the invertebrate assemblages. The similarity of the species assemblages within a spring was calculated using Bray-Curtis similarity after square root transformation. A Simper analysis was performed to assess the similarity of invertebrate assemblages within each spring 2019–2021. A similarity analysis (ANOSIM), which is analogous to an ANOVA, but relies on a similarity matrix and makes few assumptions on data, was used to test the significance of similarity within sites. Ordination was performed using a non-metric multidimensional scaling (nMDS). An nMDS does not assume a normal distribution; only ranks are compared. The distances between samples are relative and illustrate their similarities.
For the comparison of all sites, the mean abundances of three (all springs except the springs in Val Müstair) or two sampling occasions (all other sites except Qbr_Taman) were calculated for all taxa. Again, species richness (S), Shannon diversity (H’loge) and abundance (N) were calculated for each site. A redundancy analysis (RDA) was performed using the mean values of the invertebrate assemblages from all sites and the mean values of the environmental variables. Environmental variables were chosen based on forward selection. Data were log10 transformed prior to analysis. Adjustment of the variation was done using Ezekiel’s formula (Legendre et al. 2011).
RDA was performed using CANOCO 5.0 (ter Braak and Smilauer 1998); all other analyses were performed using PRIMER 7.0 (Clarke and Gorley 2006).
Results
Environmental parameters
The mean water temperature ranged from 1.5 °C in Q1_Bescha to 7.0 °C in Punt Periv (Tab. 2). Some of the sites were very stable, whereas others showed considerable variations in water temperatures. The amplitude was lowest in the Punt Periv spring (0.8) and highest in the Qbr_VF3 springbrook (10.7). The lowest minimum temperatures were recorded in Q1_Bescha and Q_Taman (0 °C). The highest maximum temperatures were reached in the Wegerhaus spring (11.1° C). The seven sites with continuous data from June 2020 to May 2023 show different degrees of variability (Fig. 1). Q_VF2, Q_Tiats and the Laider brook show a relatively high variability, while in Q_Ravit and its springbrook QBr_Ravit the water temperature varies only slightly over time. With the exception of Q_Tiats, where the water temperature decreased significantly (S = – 195, Z = – 2.6442, p = 0.00819), no significant trend of a temporal change in water temperature was observed.
Violin and box plots for the sites from which continuous water temperature data exists from June 2020 to May 2023. The horizontal line represents the median, the dark colored box is the 25–75% interquartile range, and the minimal and maximal values are shown with short horizontal lines. Smooth colored shapes represent the kernel density plot of all measured values
The physicochemical parameters varied only slightly during the entire period; especially the pH was very constant (Table 3). The EC was lowest at the uppermost sites Era da la Bescha (Q2_Bescha: 31 ± 3 µS/cm) in Val Müstair and highest in the Ravitschana sites (Q_Ravit and Qbr_Ravit: 430 ± 36 and 429 ± 35 µS/cm, respectively) in Val S-charl. All springs were saturated with oxygen; only spring Q2_Bescha had a slightly lower oxygen content. The discharge was mostly < 10 l/s, while the larger brooks had a discharge > 50 l/s. The highest discharge was measured in the brook Tiatscha Sot (95.2 ± 33.3 l/s).
Ammonia (-NH4+) and phosphate (-PO43−) concentrations were below detection limit, and fluoride (-F−) was below detection limit in some sites, mostly springbrooks. Nitrate (-NO3−) was always detectable, and concentrations varied from 0.36 ± 0.14 mg/l in QBr_VF2 to 1.60 ± 0.27 mg/l in Q_VF1. Sulfate (-SO42) concentrations were particularly high in the springs Punt Periv (77.71 ± 15.49 mg/l) and Q_Ravit (90.68 ± 20.61 mg/l) and the springbrook Qbr_Ravit (93.03 ± 22.72 mg/l) (Online Resource 2). The substrate composition was dominated by moss in many springs, while the share of sand and detritus increased downstream in the springbrooks (Online Resource 3). The brooks were dominated by coarse inorganic material. As there are no deciduous trees around the sites, no leaf litter besides spruce and pinus needles was found in the springs.
Faunistic assemblages: springs 2019–2021
The lowest number of taxa (n = 16) was found in the Wegerhaus spring in 2019 and the highest in springs Q_VF2 and VA2 in 2021 (n = 32). The variation in the number of taxa in springs is low in springs Q_VF3 and Q_Pluogls (30–31 taxa/year and 26–28 taxa/year, respectively), whereas it is high in Q_VF5 (21–31 taxa/year). The similarity of samples within as spring ranged from 57% (Q_Ravit) to 72% (Q_Taman). The percentage of taxa found in each year ranged from 30% in Punt Periv to 47% in Q_Pluogls (Table 4).
Overall, the variability of taxa found in a spring within 3 years is very high. However, some species such as Crenobia alpina, Nemoura mortoni and Protonemura lateralis occur constantly in almost every spring. Despite the variability between years within springs, significant faunistic differences were found between springs for all springs sampled in 2019, 2020 and 2021 (R: 0.817, p: 0.001) (Fig. 2).
Non-metric multidimensional scaling (nMDS) based on the faunistic data of all springs sampled three times. Black triangle = 2019, inverted gray triangle = 2020, asterisk = 2021). Distances between points are relative and illustrate similarities between samples. Transformation: square root, similarity index: Bray-Curtis similarity. Stress < 0.2 useful 2-dimensional ordination of the high-dimensional assemblage structure (Clarke & Gorley 2006). Significant faunistic differences were found between springs (R: 0.817, p: 0.001)
Faunistic assemblages: all sites
Overall, 98 species and taxa at higher taxonomic level were identified. The number of taxa per site ranged from 17 in Q1_Bescha to 47 in Q_VF2. Shannon diversity was lowest in Q1_Bescha (1.083) and highest in the brook Laider (2.693). The highest abundance was found in the springbrook Qbr_Tiats (Table 5), which is mainly due to the high density of Chironomidae, e.g., in the Wegerhaus spring. Trichoptera were by far the most species-rich order with 22 taxa identified to species level. Seven water mite species were identified, although the species-rich genus Lebertia and Atractides were only identified to genus level. Amphipoda were only found at four sites: Gammaus fossarum occurred in high densities in spring Punt Periv and was found once in Q_Pluogls. Niphargus cf. thienemanni was found in the Wegerhaus spring and in Q2_Posa (Online Resource 4).
The constrained RDA performed for all sites revealed differences between sites based on environmental variables selected by a forward selection (Monte-Carlo test: F = 2.6; P = 0.002). The selected environmental variables wood, elevation, pH and FPOM explained 34.3% of the total variance among sites (adjusted variation among sites is 21.1%). Axis 1 explained 15% of the variation, axes 2 and 3 explained 9.86 and 5.92% of the variation, respectively, and axis 4 explained 3.47% of the variation. The three significant explanatory variables accounted for 28.4% of the total variance: wood, elevation (both P < 0.005) and pH (P < 0.05) (Table 6). The first two axes indicate a separation of sites by altitude and pH and by the amount of wood and FPOM (Fig. 3).
Ordination of 25 sites and 98 taxa by redundancy analysis (RDA). The environmental parameters with the highest explanatory power in the model are indicated with arrows. Only genus and species of the most diverse taxa Trichoptera, Plecoptera and Hydrachnidia are displayed. AcrZerb Acrophylax zerberus, AllgUnct Allogamus uncatus, AmphStand Amphinemura standfussi, ApatHelv Apatania helvetica, BeraPull Beraea pullata, ConsCons Consorophylax consors, DictFont Dictyogenus fontium, DrusBigt Drusus biguttatus, DrusChrs Drusus chysotus, DrusDisc Drusus discolor, DrusMelan Drusus melanchaetes, DrusMont Drusus monticola, GamFoss Gammarus fossarum, HalsRub Halesus rubricollis, HdyrPlac Hydrovolzia placophora, HygrNorv Hygrobates norvegicus, IsopRivl Isoperla rivulorum, LeuctraGrBr Leuctra braueri group, LithNigr Lithax niger, MelMel Melampophylax melampus, MetaFlav Metanoea flavipennis, NemoMort Nemoura mortoni, NemoSinu Nemoura sinuata, NemrPict Nemurella pictetii, PartStei Partnunia steinmanni, PhilMon Philopotamus montanus, PhilVari Philopotamus variegatus, PlecGen Plectrocnemia geniculata, ProtAub Protonemura auberti, ProtBrev Protonemura brevistyla, ProtLatr Protonemura lateralis, RhyaInt Rhyacophila intermedia, RhyaTris Rhyacophila tristis, SperMutl Sperchon mutilus, SperThie Sperchon thienemanni, SperVerr Sperchonopsis verrucosa, SperViol Sperchon violaceus
High altitude sites of the “Era da la Bescha” and “Tamangur” systems have the lowest pH and EC. They are inhabited by alpine trichopteran species such as Drusus melanchaetes, Drusus monticola and Allogamus uncatus. Sites with a high share of wood are situated in Val Ftur and the Alp Champatsch in Val Müstair. They are inhabited by many spring specialists such as Drusus chrysotus, Partnunia steinmanni and Nemoura sinuata. Many of the springbrooks and also some springs had a high share of FPOM. These sites often exhibited a relatively high discharge and were inhabited by crenobiontic water mites such as Hygrobates norvegicus and Sperchon violaceus, but also by rhithral species (e.g., Protonemura brevistyla and Isoperla rivulorum). The stonefly Nemoura mortoni was especially abundant at sites with a high FPOM content. It is a crenophilic species found from the epirhithral to the eucrenal (Moog 1995).
Discussion
Conceptual framework for long-term studies
When initiating long-term monitoring, it is important to consider certain aspects that help to assert the feasibility and validity over time. Cantonati et al. (2022) gave a comprehensive overview of methodological aspects that need to be considered. Their considerations are mostly based on the long-term studies on springs in the Berchtesgaden National Park in Germany (e.g., Gerecke and Franz 2006) and in the Adamello-Brenta Nature Park in Italy (e.g., Cantonati et al. 2007). These and other routinely applied biodiversity monitoring programs are usually based on a 5-year interval for flora and fauna surveys for financial reasons (Koordinationsstelle BDM 2014). In contrast, we are confident that annual faunistic sampling is needed: as shown by the first 3 years of sampling in the UBEVM and former studies (e.g., von Fumetti 2014; von Fumetti et al. 2017), a certain variability of species assemblages due to demographic or stochastic factors is common. Variability over time should therefore not be over-interpreted. On the other hand, mid- or long-term trends in species shifts may be overlooked if samples are only taken every 5 years.
The intervals of abiotic measurements are always a trade-off between financial and time constraints and the required accuracy. In the UBEVM, we measured the physico-chemical parameters and analyzed the nutrients three times a year. We detected slight seasonal variations and recommend not to reduce the interval. Indeed, Cantonati et al. (2022) propose four or at least three measurements per year. An even more frequent sampling interval would be desirable if we want to better understand how the change of glacier melt will influence environmental conditions in springs. This also applies to discharge, which varies both during the year and between years. Changes in the discharge regime, possibly induced by climatic changes in the upcoming decades, may only be detected if we know the basic fluctuations in discharge in the investigated springs and springbrooks. However, one must always be aware that precise discharge measurements in natural springs are challenging (e.g., Frisbee et al. 2013). Discharge measurements calculated based on the flow velocity and the cross section must be generally considered as relatively inaccurate as evidenced by the high standard deviations at our sites. However, it is the best low-impact method that can be applied in springs. Therefore, the measurements must be carried out as accurately as possible on a well-defined and fixed cross-section at every site.
Map** the springs and springbrooks may be reduced to once a year. Larger intervals of up to 5 years as proposed by Cantonati et al. (2022) are not recommended. Despite being much more stable than lower sections of rivers, also springs and springbrooks are exposed to natural disturbances, and a certain redistribution of the sediments is evident. Especially in the Swiss National Park, we observe that trees around and in springs start to die off. Fallen trees can change the flow conditions and in consequence substrate composition considerably. Even trampling by chamois and red deer is apparent at some sites and should be documented as a potential source of nutrient input. Sudden heavy rainfall during thunderstorms can lead to mudflows and alter freshwater systems in the region dramatically (e.g., Mühlemann 2017, https://nationalpark.ch/natur/prozesse/). Indeed, the very species-rich springbrook Ova dals Pluogls became completely dry in July 2022 because of a mudflow.
Water temperature logging devices are very appropriate for long-term studies in springs. They deliver reliable data with high resolution with relatively low effort. Several studies on springs already applied them successfully for shorter durations, but not longer than 1 year (e.g., Küry et al. 2017; Schenková et al. 2020; Výravský et al. 2023). When using temperature loggers on a long-term basis, certain aspects need to be considered. The battery life of the loggers guarantees approximately 1.5 years of logging. Batteries should be exchanged in time so there are no gaps in the data series. To extend battery life, the logging interval may be increased to 2 hourly and potentially even a second logger can be placed in the spring. When installing temperature loggers, one should also consider the potential impact of solar radiation. Loggers only heat up minimally in summer, but in springs with a low water depth they should preferably be placed at a shaded spot.
Environmental parameters—stability versus variability
Results confirm the stability of the water temperature for most of the springs as well as a higher amplitude in the larger springbrooks and brooks. Stability of the water temperature at the spring outflow depends on the depth of the aquifer (Cantonati et al. 2006). The most stable sites Punt Periv and Ravitschana seem to be fed by a deep aquifer (Küry et al. 2017). Springs with a higher amplitude can be influenced by rapid infiltration and shallower aquifers (van der Kamp 1995), higher contributions of meltwater, e.g., from rock glaciers (Brighenti et al. 2019) or unsaturated flow, i.e., water flow in the unsaturated zone of the soil (Frisbee et al. 2013). In the spring Q1_Era da la Bescha, water temperature never exceeded 2.6 °C. Meltwater from rock glaciers might stabilize the water temperature of this spring at ~ 2.5 °C (Brighenti et al. 2019). Moreover, sudden heavy rainfall can change the water temperature considerably on a short-term basis also in springs (Küry et al. 2017).
In general, results confirm our first hypothesis that springs and springbrooks are environmentally stable. Especially pH and EC were constant and showed no change over time with only slight seasonal variations at some sites. Certain spatial differences of pH and EC can be seen: in most of the sites the pH is > 8.0 and the EC > 200 µS/cm, while in the Tamangur and Era da la Bescha sites pH and EC are much lower. Despite being mainly situated in the Engadine dolomite, it cannot be excluded that the sites at Tamangur are geologically influenced by crystalline rocks (“Sesvenna-Kristallin,” Trümpy 1997). However, these sites are at the highest altitude and might also be influenced by ice-rich permafrost (https://map.geo.admin.ch; Kenner et al. 2019). It has been shown in the Pyrenees that a contribution of glacier meltwater leads to a substantial decrease of the EC (Brown et al. 2003). In the streams Aqua and Fuorn in the SNP, on the other hand, long-term data hint at a higher EC in the stream (Fuorn) influenced by ice fields (Sertić Perić et al. 2015). However, the EC in both streams was in the mean range measured at our sites (~ 250 µS/cm) and much higher than in the springs with the lowest EC (< 70 µS/cm). Nitrate values were in the same range as measured by Robinson et al. (2008); in the Punt Periv spring, for example, almost the same values were measured in 2004, proving the overall stability of this spring. The influence of livestock, visible by eye at the springs Q_Taman, Q_Tiats and Q2_Posa, did not result in increased nitrate values. Relatively high nitrate levels were measured several times in forested springs, though, in areas where the presence of chamois and red deer in the vicinity of the springs was evident. However, an even higher nutrient input in livestock influenced springs might be masked by the presence of moss and macrophytes which take up nitrate (Thies et al. 2013) and are very abundant in the springs influenced by livestock. Rock glacier meltwater can be enriched with ions (Schlüchter et al. 2013; Thies et al. 2013), which could explain the relatively high nitrate concentrations at some sites, e.g., Q_VF1 and Q_Pluogls, as well. Nitrate concentrations of 1–2 mg/l NO3 seem to be the airborne “pollution” of alpine headwaters deriving from rainfall and meltwater input (Schlüchter et al. 2013).
Discharge measurements are rarely implemented on a regular basis in natural springs because of the aforementioned difficulties to measure discharge properly. Still, a comparison of the discharge classes according to Hoffsten and Malmqvist (2000) is possible. Most of the springs had a low discharge of < 10 l/s, which is in accordance with measurements of Robinson et al. (2008) at springs in the UBEVM and of Cantonati et al. (2020) in the Northern Apennines. An increase in the following springbrook sections was measured at some sites, but some springbrooks had a very low discharge as well. A shift of the discharge peak from mid-summer to spring is predicted for the future (Michel et al. 2022) because of the varying input of snow and glacier meltwater as well as rainfall. Discharge varied considerably throughout the year and was lowest in summer and highest in October at many sites. At some sites discharge already peaked in late May. However, due to the methodological difficulties one should interpret the seasonal fluctuations cautiously. For Switzerland, an overall decrease of the discharge in streams was shown for the past 50 years (Michel et al. 2020). A slight trend of a decrease was visible from 2019 to 2022 in some sites in the UBEVM as well. This might already hint at decreasing discharge values in the region.
Fauna of springs and springbrooks
Similar to the environmental parameters a certain variability of the faunistic assemblages was evident, while stability prevailed over time. This is in accordance with our second hypothesis and other studies comparing the fauna in springs over time (Gerecke et al. 2006; von Fumetti 2014; von Fumetti et al. 2017). Similarity of the faunistic assemblages within the springs from 2019 to 2021 was generally much higher than in springs in the Bernese Alps (von Fumetti et al. 2017). In the SNP, Knispel and Lubini (2015) found no considerable change of the Plecopteran fauna in the headwaters including springs throughout the past 60 years. Our sampling in 2019–2021 confirms that springs are stable habitats with usually low disturbance frequency and intensity. However, as mentioned above, mudflows can be catastrophic events, suddenly destroying brooks and their biota. Despite overall stability, the observed fluctuations between years show that sampling every 5 years would not display the spring fauna properly. Due to seasonal fluctuations, we already miss species in the one or the other year when only sampling once a year. Even though emerging insects have a distinct seasonal pattern (Gerecke et al. 2009), the exceptionally cool and wet weather in spring 2021 (MeteoSchweiz 2022) possibly slowed down larval development.
One goal of the long-term approach initiated in 2019 is to investigate possible changes of the distribution of species along the longitudinal gradient of streams. In the past decades, a shift in the species composition of aquatic macroinvertebrates occurred in lowland and low mountain ranges (Jourdan et al. 2018): Plecoptera are vulnerable to climatic changes, while Ephemeroptera could even profit from a warming. For alpine regions, a high vulnerability of cold stenothermal and crenobiontic species is generally predicted (Küry et al. 2018). So far, most springs and corresponding springbrooks investigated in the UBEVM are inhabited by very similar species assemblages. The springbrooks are more species rich as also rhithrobiontic species such as Protonemura brevistyla co-occur with crenophilic and crenobiontic species. Rhithrobiontic species such as Isoperla rivulorum mostly occurred at sites with FPOM and sand and a higher discharge, while crenobiontic species such as Partnunia steinmanni prefer wooded springs. Due to the different substrate compositions of springs and springbrooks and the increasing discharge downstream, we conclude that that rhithrobiontic species will probably not colonize springs and replace crenobiontic species. This has to be confirmed with ongoing monitoring in the upcoming decades.
Elevational differences were another factor influencing species composition in springs in the UBEVM. Sites within the forest are much more shaped by organic substrates such as wood and moss. These are preferred substrates of typical crenobiontic taxa such as certain water mite species. This indicates that the tree line is a natural barrier for crenobiontic species in the Alps (Wigger et al. 2015; von Fumetti and Blattner 2017). A reforestation might therefore be important for a shift of the species composition in springs in alpine regions; spring specialists could even profit from a rising treeline and rising water temperatures as long as the springs do not fall dry.
Among the water mites many crenobiontic species exist (e.g., Blattner et al. 2019). Hydrachnidia often have very specific demands concerning substratum composition (Di Sabatino et al. 2000) and are indicators for spring habitat stability (Gerecke and Martin 2006). They are very well studied in the UBEVM (e.g., Bader 1975; Blattner et al. 2022) and therefore of special importance when addressing possible changes of the species assemblages over time. Chironomidae and also other Dipteran taxa are very abundant in springs and springbrooks. Among the Chironomidae, glacial specialists and cold-stenothermal taxa exist, which are adapted to the harsh environments in glacially influenced headwaters (e.g., Füreder et al. 2001; Niedrist and Füreder 2016). Inclusion of chironomids in our spring monitoring would be very important as many crenobiontic chironomid species exist as well: in Alpine and pre-Alpine springs in Italy, 37% of all Chironomidae identified in 81 springs were crenophilous-crenobiontic species (Lencioni et al. 2011). Although it is not our goal to consider the whole spring biota in our long-term approach, the inclusion of all Hydrachnidia on species level and Diptera would therefore be a strong improvement. To overcome the dilemma of overly demanding classical species identification, we implemented an eDNA-Metabarcoding approach in 2022. Single species detection of target species works well (Blattner et al. 2021) for springs, and concepts exist for implementing eDNA approaches in routinely applied freshwater monitoring programs (e.g., Fernandez et al. 2018; Pawlowski et al. 2020). Springs are exceptionally well suited for eDNA metabarcoding as one can be sure that the sequenced DNA originates from the spring and not from another river section. Despite being a method still in its infancy, eDNA metabarcoding is therefore already used for monitoring springs by biosphere reserves, national parks and federal authorities in Europe (e.g., https://www.bvd.be.ch/de/start/themen/wasser/gewaesserqualitaet/quellen-als-lebensraum.html). By including eDNA we are now able to (1) include taxonomically demanding taxa in the analysis and (2) sample the macroinvertebrates in a much less invasive way.
Conclusion
All measured variables, including species assemblages, show a certain variability but prove overall stability in the environmental variables and species composition. The current paper therefore provides a solid baseline of the environmental conditions and species assemblages in springs and springbrooks in the Alps, which possibly will change in the upcoming decades because of climate change. From these first years of monitoring in the UBEVM, we can conclude that protected areas are very suitable for long-term studies, but a certain degree of anthropogenic impact is evident nonetheless because of extensive farming activities. Moreover, natural disturbance events such as mudflows can extinguish life everywhere, and a loss of monitoring sites must always be accepted. Continuous monitoring of all relevant environmental parameters and the faunistic assemblages is inevitable to detect possible changes over time. To close the “monitoring gap” of particularly the Dipterans and to overcome the risk of “over-sampling” a spring when sampling every year, the implementation of an eDNA metabarcoding was important. In the future, 'classical' faunistic sampling will be carried out every 4 years, with eDNA metabarcoding serving as a non-invasive method in between. Intensified measurements of the abiotic parameters at selected sites will help to better understand the influence of glacier and permafrost meltwater. With this solid approach, we will be able to observe climate-induced changes in alpine springs, springbrooks and brooks on a long-term basis. This will also be of high value for other regions and freshwater habitats.
Data availability
Data are available from the authors upon reasonable request.
References
Amann E, Brandstetter CM, Kapp A (1994) Käfer am Wasser: Gattungsschlüssel der (semi)aquatischen Käfer Mitteleuropas. Erster Voralberger Coleopterologischer Verein, Bürs/Vorarlberg.
Aubert J (1965) Les Plécoptères du Parc National Suisse. Ergebnisse der Wissenschaftlichen Untersuchungen Im Schweizerischen Nationalpark 55:221–271
Bader C (1975) Die Wassermilben des Schweizerischen Nationalparks. Ergebnisse der wissenschaftlichen Untersuchungen im Schweizerischen Nationalpark 14
Bässler C, Müller J, Hothorn T, Kneib T, Badeck F, Dziock F (2010) Estimation of the extinction risk for high-montane species as a consequence of global warming and assessment of their suitability as cross- taxon indicators. Ecol Ind 10:341–352. https://doi.org/10.1016/j.ecolind.2009.06.014
Biggs J, Von Fumetti S, Kelly-Quinn M (2017) The importance of small water bodies for biodiversity and ecosystem services: implications for policy makers. Hydrobiologia 793:3–39. https://doi.org/10.1007/s10750-016-3007-0
Blattner L, Gerecke R, von Fumetti S (2019) Hidden biodiversity revealed by integrated morphology and genetic species delimitation of spring dwelling water mite species (Acari, Parasitengona: Hydrachnidia). Parasit Vectors 12:492. https://doi.org/10.1186/s13071-019-3750-y
Blattner L, Ebner J, Zopfi J, von Fumetti S (2021) Targeted non-invasive bioindicator species detection in eDNA water samples to assess and monitor the integrity of vulnerable alpine freshwater environments. Ecol Ind 129:107916. https://doi.org/10.1016/j.ecolind.2021.107916
Blattner L, Lucek K, Beck N, Berner D, von Fumetti S (2022) Intra-Alpine Islands: Population genomic inference reveals high degree of isolation between freshwater spring ecosystems. Divers Distrib 28:291–305. https://doi.org/10.1111/ddi.13461
Brighenti S, Tolotti M, Bruno MC, Wharton G, Pusch MT, Bertoldi W (2019) Ecosystem shifts in Alpine streams under glacier retreat and rock glacier thaw: A review. Sci Total Environ 675:542–559. https://doi.org/10.1016/j.scitotenv.2019.04.221
Brown LE, Hannah DM, Milner AM (2003) Alpine stream habitat classification: an alternative approach incorporating the role of dynamic water source contribution. Arct Antarct Alp Res 35:313–322
Bundesamt für Umwelt (1998) Methoden zur Untersuchung und Beurteilung der Fliessgewässer in der Schweiz: ModuI-Stufen-Konzept. Bundesamt für Umwelt, Bern. Mitteilungen zum Gewässerschutz 26
Bundesamt für Umwelt (2019) Methoden zur Untersuchung und Beurteilung von Fliessgewässern (IBCH_2019). Makrozoobenthos – Stufe F. Bundesamt für Umwelt, Bern. Umwelt-Vollzug Nr. 1026
Cantonati M, Gerecke R, Bertuzzi E (2006) Springs of the Alps – sensitive ecosystems to environmental change: from biodiversity assessments to long-term studies. Hydrobiologia 562:59–96. https://doi.org/10.1007/s10750-005-1806-9
Cantonati M, Bertuzzi E, Spitale D (2007) The spring habitat: Biota and sampling methods. Monografie del Museo Tridentino di Scienze Naturali 4
Cantonati M, Segadelli S, Spitale D, Gabrieli J, Gerecke R, Angeli N, De Nardo MT, Ogata K, Wehr JD (2020) Geological and hydrochemical prerequisites of unexpectedly high biodiversity in spring ecosystems at the landscape level. Sci Total Environ 740:140157. https://doi.org/10.1016/j.scitotenv.2020.140157
Cantonati M, Lichtenwöhrer K, Leonhardt G, Seifert L, Mustoni A, Hotzy R, Schubert E, Blattner L, Bilous O, Lotz A, Poschlod B, Gerecke R (2022) Using springs as sentinels of climate change in nature parks north and south of the Alps: a critical evaluation of methodological aspects and recommendations for long-term monitoring. Water 14:2843. https://doi.org/10.3390/w14182843
Clarke KR, Gorley RN (2006) PRIMER v6: user manual. PRIMER-E, Plymouth
Di Sabatino A, Gerecke R, Martin P (2000) The biology and ecology of lotic water mites (Hydrachnidia). Freshw Biol 44:47–62. https://doi.org/10.1046/j.1365-2427.2000.00591.x
Fattorini S, Borges PAV, Fiasca B, Galassi DMP (2016) Trapped in the web of water: groundwater-fed springs are island-like ecosystems for the meiofauna. Ecol Evol. https://doi.org/10.1002/ece3.2535
Fernandez S, Rodriguez S, Martinez JL, Borrell YJ, Ardura A, Garcia-Vazquez E (2018) Evaluating freshwater macroinvertebrates from eDNA metabarcoding: A river Nalon case study. PLoS ONE 13:e0201741. https://doi.org/10.1371/journal.pone.0201741
Frisbee MD, Philipps FM, White AF, Campbell AR, Liu F (2013) Effect of source integration on the geochemical fluxes from springs. Appl Geochem 28:32–54. https://doi.org/10.1016/j.apgeochem.2012.08.028
Füreder L, Schütz C, Wallinger M, Burger R (2001) Physico-chemistry and aquatic insects of a glacier-fed and a spring-fed alpine stream. Freshw Biol 46:1673–1690. https://doi.org/10.1046/j.1365-2427.2001.00862.x
Gerecke R, Franz H, Cantonati M (2009) lnvertebrate diversity in springs of the National Park Berchtesgaden (Germany): relevance for long-term monitoring. Verh Int Ver Theor Angew Limnol 30(8):1229–1233. https://doi.org/10.1080/03680770.2009.11923918
Gerecke R, Cantonati M, Spitale D, Stur E, Wiedenbrug S (2011) The challenges of long-term ecological research in springs in the northern and southern Alps: Indicator groups, habitat diversity, and medium term change. J Limnol 70:168–187. https://doi.org/10.3274/JL11-70-S1-13
Gerecke R, Franz H (2006) Quellen im Nationalpark Berchtesgaden. Lebensgemeinschaften als Indikatoren des Klimawandels. Nationalpark Berchtesgaden, Forschungsbericht 51
Gerecke R, Martin P (2006) Spinnentiere: Milben (Chelicerata: Acari). In: Gerecke R, Franz H (eds) Quellen im Nationalpark Berchtesgaden, Forschungsbericht 51:122–149
Gerecke R, Schrankel I, Stur E, Wagner R, Wiedenbrug S (2006) Die Langzeituntersuchungsstellen: Feinverteilung der Fauna und erste Beobachtungen zu Veränderungen in der Zeit. In: Gerecke R, Franz H (eds) Quellen im Nationalpark Berchtesgaden, Forschungsbericht 51:221–254.
Glöer P (2017) Süsswassermollusken. Ein Bestimmungsschlüssel für die Muscheln und Schnecken im Süsswasser der Bundesrepublik Deutschland. Senser-Druck, Augsburg
Haller H, Eisenhut A, Haller R (2013) Atlas des Schweizerischen Nationalparks - die ersten 100 Jahre: Bd. 99/I. Haupt, Bern
Hering D, Schmidt-Kloiber A, Murphy J, Lücke S, Zamora-Munoz C, Lopez-Rodriguez MJ, Huber T, Graf W (2009) Potential impact of climate change on aquatic insects: a sensitivity analysis for European caddisflies (Trichoptera) based on distribution patterns and ecological preferences. Aquat Sci 71:3–14. https://doi.org/10.1007/s00027-009-9159-5
Hoffsten P-O, Malmqvist B (2000) The macroinvertebrate fauna and hydrogeology of springs in central Sweden. Hydrobiologia 436:91–104
Hogan HSC, O’Sullivan JJ, Bruen M, Jarvie HP, Cox EJ, Bowes MJ, Kelly-Quinn M (2023) A review of the nature and source of nutrient impairment in small streams: a desk-based characterisation for targeted mitigation measures. Hydrobiologia 850:3293–3311. https://doi.org/10.1007/s10750-022-05114-1
Illies J (1961) Versuch einer allgemeinen biozönotischen Gliederung der Fliessgewässer. – Internat. Rev Ges Hydrobiol 46:205–213
Illies J, Botosaneanu L (1963) Problèmes et méthodes de la classification et de la zonation écologique des eaux courantes, considérées surtout du point de vue faunistique. – Mitt. Internat Verein Limnol 12:1–57
Jourdan J, O’Hara RB, Bottarin R, Huttunen K-L, Kuemmerlen M, Monteith D, Muotka T, Ozoliņš D, Paavola R, Pilotto F, Springe G, Skuja A, Sundermann A, Tonkin JD, Haase P (2018) Effects of changing climate on European stream invertebrate communities: a long-term data analysis. Sci Total Environ 621:588–599. https://doi.org/10.1016/j.scitotenv.2017.11.242
Keller F (2013) Permafrost. Klimasignale aus dem Untergrund. In: Haller H, Eisenhut A, Haller RM (eds) Atlas des Schweizerischen Nationalparks – die ersten 100 Jahre. Haupt, Bern, pp 32–33
Kenner R, Noetzli J, Hoelzle M, Raetzo H, Phillips M (2019) Distinguishing ice-rich and ice-poor permafrost to map ground temperatures and ground ice occurrence in the Swiss Alps. Cryosphere 13:1925–2194. https://doi.org/10.5194/tc-13-1925-2019
Knispel S, Lubini V (2015) Assessing the stability of stonefly (Plecoptera) biodiversity in the Swiss National Park. Mitt Schweiz entomol Ges 88:257–271. https://doi.org/10.5281/zenodo.33951
Koordinationsstelle BDM (2014) Biodiversitätsmonitoring Schweiz BDM. Beschreibung der Methoden und Indikatoren. Bundesamt für Umwelt, Bern. Umwelt-Wissen 1410:104
Küry D, Lubini V, Stucki P (2017) Temperature patterns and factors governing thermal response in high elevation springs of the Swiss Central Alps. Hydrobiologia 793:185–197. https://doi.org/10.1007/s10750-016-2918-0
Küry D, Lubini V, Stucki P (2018) Verletzlichkeit von Eintagsfliegen, Steinfliegen und Köcherfliegen alpiner Quellen gegenüber Klimaveränderungen. Jahrbuch Des Vereins Zum Schutz der Bergwelt München 83:199–218
Legendre P, Oksanen J, ter Braak CJF (2011) Testing the significance of canonical axes in redundancy analysis. Methods Ecol Evol 2:269–277. https://doi.org/10.1111/j.2041-210X.2010.00078.x
Lencioni V, Marziali L, Rossaro B (2011) Diversity and distribution of chironomids (Diptera, Chironomidae) in pristine Alpine and pre-Alpine springs (Northern Italy). J Limnol 70:106–121. https://doi.org/10.3274/JL11-70-S1-08
Lencioni V, Stella E, Zanoni MG, Bellin A (2022) On the delay between water temperature and invertebrate community response to warming climate. Sci Tot Environ 837:155759. https://doi.org/10.1016/j.scitotenv.2022.155759
Liess M, Böhme A, Gröning J, Liebmann L, Lück M, Reemtsma T, Römerscheid M, Schade U, Schwarz B, Vormeier P, Weisner O (2023) Belastung von kleinen Gewässern in der Agrarlandschaft mit Pflanzenschutzmittel-Rückständen – TV1 Datenanalyse zur Pilotstudie Kleingewässermonitoring 2018/2019. Umweltbundesamt, Dessau
Lubini V, Knipsel S, Vinçon G (2012) Die Steinfliegen der Schweiz: Bestimmung und Verbreitung. Fauna Helvetica, 27. Schweizerische Entomologische Gesellschaft, Neuchatel
Lubini V, Stucki P, Vicentini H, Küry D (2014) Bewertung von Quell-Lebensräumen in der Schweiz. Entwurf für ein strukturelles und faunistisches Verfahren. Bericht im Auftrag des Bundesamtes für Umwelt BAFU
MeteoSchweiz (2022) Klimabulletin Jahr 2021. Zürich
Michel A, Brauchli T, Lehning M, Schaefli B, Huwald H (2020) Stream temperature and discharge evolution in Switzerland over the last 50 years: annual and seasonal behavior. Hydrol Earth Syst Sci 24:115–142. https://doi.org/10.5194/hess-24-115-2020
Michel A, Schaefli B, Wever N, Zekollari H, Lehning M, Huwald H (2022) Future water temperature of rivers in Switzerland under climate change investigated with physics-based models. Hydrol Earth Syst Sci 26:1063–1087. https://doi.org/10.5194/hess-26-1063-2022
Moog O (1995). Fauna Aquatica Austriaca. Wasserwirtschaftskataster, Bundesministerium für Land- und Forstwirtschaft, Wien.
Mühlemann L (2017) Quellen und Wasseraustritte im Tal der Clemgia im Unterengadin (GR). Master thesis, University of Basel
Nadig A (1942) Hydrobiologische Untersuchungen in Quellen des Schweizerischen Nationalparks im Engadin. Ergebnisse der wissenschaftlichen Untersuchungen im Schweizerischen Nationalpark 1.
Niedrist GH, Füreder L (2016) Towards a definition of environmental niches in alpine streams by employing chironomid species preferences. Hydrobiologia 781:143–160. https://doi.org/10.1007/s10750-016-2836-1
Niedrist GH, Füreder L (2023) Disproportional vulnerability of mountain aquatic invertebrates to climate change effects. Arct Antarct Alp Res 55:2181298. https://doi.org/10.1080/15230430.2023.2181298
Pawlowski J, Apothéloz-Perret-Gentil L, Mächler E, Altermatt F (2020) Anwendung von eDNA-Methoden in biologischen Untersuchungen und bei der biologischen Bewertung von aquatischen Ökosystemen. Bundesamt für Umwelt, Bern. Umwelt-Wissen 2010
Robinson CT, Schmid D, Svoboda M, Bernasconi SM (2008) Functional measures and food webs of high elevation springs in the Swiss Alps. Aquat Sci 70:432–445. https://doi.org/10.1007/s00027-008-8125-y
Schenková J, Polášková J, Bílková M, Bojková J, Syrovátka V, Polášek M, Horsák M (2020) Climatically induced temperature instability of groundwater-dependent habitats will suppress cold-adapted Clitellata species. Int Rev Hydrobiol 105:85–93. https://doi.org/10.1002/iroh.201902006
Schlüchter C, Clausen M, Haemmig C, Margreth A, Pointner E, Steiner B, Strasky S, Vetter H (2013) Quellen. Natürliche Wasseraustritte im Gelände. In: Haller H, Eisenhut A, Haller RM (eds) Atlas des Schweizerischen Nationalparks – die ersten 100 Jahre. Haupt, Bern, pp 30–31
Sertić Perić M, Jolidon C, Uehlinger U, Robinson CT (2015) Long-term ecological patterns of alpine streams: an imprint of glacial legacies. Limnol Oceanogr 60:992–1007. https://doi.org/10.1002/lno.10069
Studemann D, Landolt P, Sartori M, Hefti D, Tomka I (1992) Ephemeroptera. Insecta Helvetica. Mauron + Tinguely & Lachat SA, Fribourg
ter Braak CJF, Smilauer P (1998) CANOCO reference manual and user’s guide to Canoco for windows: software for canonical community ordination (version 5). Microcomputer Power, Ithaca
Thies H, Nickus U, Tolotti M, Tessadri R, Krainer K (2013) Evidence of rock glacier melt impacts on water chemistry and diatoms in high mountain streams. Cold Reg Sci Technol 96:77–85. https://doi.org/10.1016/j.coldregions.2013.06.006
Timoner P, Marle P, Castella E, Lehmann A (2020) Spatial patterns of mayfly, stonefly and caddisfly assemblages in Swiss running waters in the face of global warming. Ecography 43:1–14. https://doi.org/10.1111/ecog.04808
Trümpy B, Schmid SM, Conti P, Froitzheim N (1997) Erläuterungen zur Geologischen Karte 1: 5 0000 des Schweizerischen Nationalparks. Nationalpark-Forschung in der Schweiz 87:43
Van der Kamp RO (1995) The hydrogeology of springs in relation to the biodiversity of spring fauna: a review. J Kansas Entomol Soc 68:4–17
Von Fumetti S (2014) Naturnahe Quellen und ihre Lebensgemeinschaften - zehn Jahre Forschung im Röserental bei Liestal. Regio Basiliensis 55:101–114
Von Fumetti S, Blattner L (2017) Faunistic assemblages of natural springs in different areas in the Swiss National Park—a small-scale comparison. Hydrobiologia 793:175–184. https://doi.org/10.1007/s10750-016-2788-5
Von Fumetti S, Felder S (2014) Faunistic characterization of Alpine springs in the Swiss National Park. Eco.mont 6:23–29
Von Fumetti S, Wigger FW, Nagel P (2017) Temperature variability and its influence on macroinvertebrate assemblages of alpine springs. Ecohydrology. https://doi.org/10.1002/eco.1878
Výravský D, Klímová Hřívová D, Bojková J, Horsák M, Zhai M (2023) Effects of thermal stability on microcrustacean assemblages in spring fens. Inland Waters 13:86–100. https://doi.org/10.1080/20442041.2022.2139585
Waringer J, Graf W (2011) Atlas der mitteleuropäischen Köcherfliegenlarven. Erik Mauch Verlag, Dinkelscherben
Wigger FW, Schmidlin L, Nagel P, von Fumetti S (2015) Macroinvertebrate assemblages of natural springs along an altitudinal gradient in the Bernese Alps, Switzerland. Ann Limnol 51:237–247. https://doi.org/10.1051/limn/2015018
Acknowledgements
We thank the Swiss Academy of Natural Science (SCNAT), the UBEVM and the Fundaziun Pro Terra Engiadina for financial support. The analysis of the ions and nutrients was done by Judith Kobler-Waldis of the laboratory of the Environmental Geosciences of the University of Basel. Many thanks go to numerous people who helped during field work and faunistic sample sorting.
Funding
Open access funding provided by University of Basel.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Stefanie von Fumetti. The first draft of the manuscript was written by Stefanie von Fumetti, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article. Partial financial support was received from the Swiss Academy of Natural Science (SCNAT), the UNESCO Biosphere Reserve Engiadina Val Müstair (UBEVM) and the Foundation Pro Terra Engiadina.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
von Fumetti, S., Aberhalden, A. Monitoring potential impacts of climate change on the biodiversity of springs and springbrooks in the Central Alps. Aquat Sci 86, 80 (2024). https://doi.org/10.1007/s00027-024-01095-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-024-01095-6