Search
Search Results
-
Establishment of the African Medicines Agency: progress, challenges and regulatory readiness
Insufficient access to quality, safe, efficacious and affordable medical products in Africa has posed a significant challenge to public health for...

-
Evaluating the Success of ZaZiBoNa, the Southern African Development Community Collaborative Medicines Registration Initiative
The Southern African Development Community (SADC) collaborative medicines registration initiative ZaZiBoNa is a successful regional work-sharing...
-
Product Design
Pharmaceutical companies, hospital and community pharmacies prepare medicines. Although batch sizes vary greatly, the underlying principles of...
-
Evidence in Evaluation Research
The introduction of a new health technology (e.g., drug, device, vaccine, complex interventions, systems) always repercuss on the clinical flow,...
-
Demonstrating that Real World Evidence Is Fit-For-Purpose to Support Labeling: Parallels to Patient Reported Outcomes in the Pursuit of Labeling Claims
IntroductionIn December 2021, U.S. Food & Drug Administration (FDA) will issue guidance on the use of real-world evidence (RWE) to support new...

-
New Approaches to Regulatory Innovation Emerging During the Crucible of COVID-19
The urgency and impact of the ongoing COVID-19 pandemic are changing global drug development and regulatory processes. The need for speed to...
-
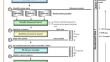
An evaluation of the Caribbean regulatory system centralized assessment process for medicines submitted 2017–2018 using the OpERA methodology
BackgroundThe Caribbean Regulatory System is a centralized medicine assessment procedure established to serve the needs of the Member States of the...
-
Expert Commentary: Diverse Meanings of Regulatory “Convergence”
The term regulatory “convergence” is apparently given many meanings in the field of medical products. Originally defined as a process of making...
-
Industrial Pharmacy: The Way to Create a Product (Monograph Review)
The recently published monograph “Industrial Pharmacy: The Way to Create a Product” is devoted to topical problems in this field. The unique...
-
Regulatory Reform Outcomes and Accelerated Regulatory Pathways for New Prescription Medicines in Australia
National Regulatory Authorities (NRAs) globally are facing the challenge of evaluating pharmaceutical products in a speedy manner, whilst...

-
Unrealized potential of drug repositioning in Europe during COVID-19 and beyond: a physician's perspective
Drug repositioning is the scientific strategy of investigating existing drugs for additional clinical indications. The advantages of drug...

-
Validation of Chromatographic Methods: Determining the Limit of Quantification in Practice
Key issues related to determining the limit of quantification (LOQ) in practice are considered. Requirements to the value of LOQ and its precision...

-
Co-Development of Oncology Drugs and Companion Diagnostics: Analyses of Approval Lags and Drug Development Periods in Recently Approved Cases in Japan
BackgroundThe utilization of biomarkers has become increasingly active to enhance efficiency of clinical development. This study evaluated the...

-
Pharma Collaboration for Transparent Medical Information (phactMI™) Benchmark Study: Trends, Drivers, and Value of Product Support Activities, Key Performance Indicators, and Other Medical Information Services: Insights from a Survey of 27 US Pharmaceutical Medical Information Departments
BackgroundA benchmarking survey was conducted by the phar maceutical c ollaboration for t ransparent M edical I nformation (phactMI™) consortium, with...

-
Pharma Collaboration for Transparent Medical Information (phactMI™) Benchmark Study: Results of Organizational Structure and Resourcing of Medical Information Services in Support of Building Departmental Strategies
BackgroundAn opportunity exists to leverage the collective insights and experiences of phactMI™ member companies on current medical information (MI)...

-
A Baseline Analysis of Regulatory Review Timelines for ANVISA: 2013–2016
BackgroundThe Brazilian health regulatory agency (Agência Nacional de Vigilância Sanitária, ANVISA) has embarked on transformational initiatives to...

-
Physicochemical and Technological Characteristics of Lycopus Europaeus L. Dry Herbal Extract and Related Compositions
The physicochemical and technological properties of Lycopus europaeus dry herbal extract were studied. Excipients were also selected. The...
-
Validation of Related-Substances Determination Methods for Detecting Unidentified Substances (A Review)
The review justifies the need to validate the linearity, relative accuracy, and precision of Related Substances methods for detecting unidentified...

-
How Do Drug Regulatory Bodies Deal With Potential Innovative Therapies?
Given the extensive development of new molecules over the last 10 years, regulatory authorities (RAs) have been intensively working on evaluating how...
-
How 4 Companies Became One: Co-development Under an Outsourced Model With Focus on Phase 3 Analysis and Reporting Deliverables
With the growth in co-development deals between pharmaceutical companies and the increased use of contract research organizations (CROs) in drug...
