Search
Search Results
-
Use of patient-reported outcome measures for oncology drugs receiving accelerated approval
Patient-reported outcomes (PROs) represent an important evaluation of health-related quality of life that has become more commonly incorporated into...
-
Special FDA designations for drug development: orphan, fast track, accelerated approval, priority review, and breakthrough therapy
BackgroundOver the past decades, US Congress enabled the US Food and Drug Administration (FDA) to facilitate and expedite drug development for...

-
Aspiring to Reasonableness in Accelerated Approval: Anticipating and Avoiding the Next Aducanumab
The US Food and Drug Administration’s decisions about drug approval—though guided by science, as well as relevant statutes, regulations, and guidance...

-
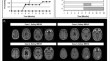
Initial Experiences with Amyloid-Related Imaging Abnormalities in Patients Receiving Aducanumab Following Accelerated Approval
Aducanumab is the first FDA-approved amyloid-lowering immunotherapy for Alzheimer’s disease. There is little real-world data to guide management of...
-
Estimating the Potential Benefits of Confirmatory Trials for Drugs with Accelerated Approval: A Comprehensive Value of Information Framework
BackgroundThe US Food and Drug Administration's Accelerated Approval (AA) policy provides a pathway for patients to access potentially life-saving...

-
Pirtobrutinib: First Approval
Pirtobrutinib (Jaypirca TM ), a highly selective, non-covalent, reversible Bruton’s tyrosine kinase (BTK) inhibitor, is being developed by Eli Lilly...

-
Resmetirom: First Approval
Resmetirom (Rezdiffra™) is an oral thyroid hormone receptor-β (THR-β) agonist being developed by Madrigal Pharmaceuticals, Inc., to target the key...

-
Talquetamab: First Approval
Talquetamab (talquetamab-tgvs; TALVEY ® ), a humanized, bispecific G-protein coupled receptor family C group 5 member D (GPRC5D)-directed CD3 T-cell...

-
Retifanlimab: First Approval
Retifanlimab (retifanlimab-dlwr; ZYNYZ TM ) is a programmed cell death 1 receptor-blocking antibody that is being developed by Incyte Corporation for...

-
Elranatamab: First Approval
Elranatamab (elranatamab-bcmm; ELREXFIO™) is a bispecific B-cell maturation antigen (BCMA)-directed CD3 T cell engager being developed by Pfizer for...

-
Sparsentan: First Approval
Sparsentan (FILSPARI™) is an oral, dual endothelin angiotensin receptor antagonist that is being developed by Travere Therapeutics for the treatment...

-
Lecanemab: First Approval
Lecanemab (lecanemab-irmb; LEQEMBI™) is a humanized immunoglobulin gamma 1 (IgG1) against aggregated soluble and insoluble forms of amyloid-β...

-
Use of real-world evidence to support regulatory decisions on medical devices in China and a unique opportunity to gain accelerated approval in “Boao Lecheng Pilot Zone”
This article aims to summarize the development and challenges of real-world data (RWD) and real-world evidence (RWE) in China and introduce a unique...
-
Delandistrogene Moxeparvovec: First Approval
Delandistrogene moxeparvovec (delandistrogene moxeparvovec-rokl; ELEVIDYS ® ) is an adeno-associated virus (AAV) vector-based gene therapy designed to...
-
Bosutinib: Pediatric First Approval
Bosutinib (BOSULIF ® ), an orally administered BCR–ABL tyrosine kinase inhibitor (TKI) developed by Pfizer Inc., is well established in the EU and the...

-
Adagrasib: First Approval
Adagrasib (KRAZATI™) is an orally available, potent, irreversible, small molecule inhibitor of KRAS G12C mutant isoform being developed by Mirati...

-
HTA Barriers for Conditional Approval Drugs
BackgroundConditional approval pathways facilitate accelerated marketing authorisation based on immature clinical evidence for drugs that address an...

-
Tofersen: First Approval
Tofersen (Qalsody ™ ) is an antisense oligonucleotide being developed by Biogen for the treatment of amyotrophic lateral sclerosis (ALS). On 25 April...

-
Ritlecitinib: First Approval
Ritlecitinib (LITFULO ™ ), an orally administered kinase inhibitor, is being developed by Pfizer for the treatment of alopecia areata, vitiligo,...

