Search
Search Results
-
New stability indicating RP-HPLC-PDA method for determination of mifepristone in bulk and tablet formulation
BackgroundMifepristone is progestational and glucocorticoid hormone antagonist. The objective of this study is to develop simple and economical...

-
Development and validation of a stability-indicating RP-HPLC method for estimation of Lefamulin in pure and pharmaceutical drug products
BackgroundCommonly occurring serious lung parenchymal infection is community-acquired bacterial pneumonia (CABP). Lefamulin acetate drug products are...

-
Stability-indicating RP-HPLC method development and validation for estimation of Mupirocin calcium in bulk and in pharmaceutical formulation
BackgroundA simple, rapid, sensitive and selective stability-indicating (RP-HPLC) method is suggested for the determination of Mupirocin calcium in...

-
Comparative study of UV spectroscopy, RP-HPLC and HPTLC methods for quantification of antiviral drug lamivudine in tablet formulation
BackgroundIn the current study, estimation of lamivudine (LMU) by UV spectroscopy, reverse-phase HPLC (RP-HPLC) and HPTLC methods in tablet...

-
Stability-indicating RP-HPLC method development and validation for simultaneous estimation of telmisartan and rosuvastatin calcium in bulk and in tablet dosage form
BackgroundThe stability-indicating chromatographic method was developed and validated for simultaneous estimation of telmisartan and rosuvastatin...

-
A single robust stability-indicating RP-HPLC analytical tool for apigenin quantification in bulk powder and in nanoliposomes: a novel approach
BackgroundApigenin (4′, 5, 7-trihydroxyflavone), a flavonoid, is present usually in fruits and vegetables possessing numerous biological properties...

-
Design of experiment-driven stability-indicating RP-HPLC method for the determination of tofacitinib in nanoparticles and skin matrix
BackgroundTofacitinib—an oral JAK inhibitor—has been recently approved by US FDA to treat moderate to severe RA. The delivery of tofacitinib to...

-
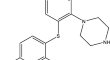
A novel stability-indicating method for determination of a new antidepressant effect of vortioxetine in a pharmaceutical formulation by using RP-HPLC
BackgroundA novel rapid, accurate, and stability-indicating reversed-phase high performance liquid chromatographic (RP-HPLC) and first derivative...
-
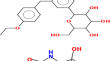
Development and validation of stability-indicating RP-HPLC method for the simultaneous determination of ertugliflozin pidolate and metformin hydrochloride in bulk and tablets
BackgroundIn the present study, an improved simple, specific, rapid, sensitive, precise, accurate and stability-indicating RP-HPLC method for the...
-
Concurrent estimation of lamivudine, tenofovir disoproxil fumarate, and efavirenz in blended mixture and triple combination tablet formulation by a new stability indicating RP-HPLC method
BackgroundAn easy, defined, rapid, and accurate reverse phase high-performance liquid chromatography method was developed and subsequently validated...

-
Experimental design approach, screening and optimization of system variables, analytical method development of flurbiprofen in nanoparticles and stability-indicating methods for high-pressure liquid chromatography
BackgroundThe development of chromatographic method and the validation of a sensitive, simple, efficient, and reversed-phase high-performance liquid...

-
Development and validation of stability-indicating RP-HPLC method for estimation of dalfampridine in bulk drug and tablet dosage form
BackgroundIn the current study, a simple, improved, precise, rapid, and accurate reverse phase liquid chromatographic method was produced for the...

-
Development and validation by statistical treatment of stability indicating RP-HPLC method for quantification of Orlistat in Orlistat-loaded solid dispersion
BackgroundMost of the analytical methods reported for the estimation of Orlistat were complex, expensive, and deficient in reproducibility with no or...

-
A new stability-indicating RP-HPLC method for the determination of dicyclomine hydrochloride and dimethicone combination in tablet dosage forms
BackgroundWe describe a “stability-indicating liquid chromatography” technique for the estimation of dimethicone (DEC) and dicyclomine hydrochloride...

-
Development and validation of stability-indicating RP-HPLC method for the simultaneous estimation of xylometazoline hydrochloride and ipratropium bromide from nasal spray dosage form
BackgroundA simple, robust, precise, and an accurate HPLC method was established for simultaneous estimation of xylometazoline hydrochloride and...

-
Stability-indicating HPLC-DAD method for the determination of empagliflozin
BackgroundA stability-indicating RP-HPLC method was developed and validated for the estimation of empagliflozin drug and its tablet dosage form using...

-
A new validated stability-indicating RP-HPLC method for simultaneous quantification of dolutegravir and lamivudine in bulk and pharmaceutical dosage form
BackgroundA fresh selective, rapid, accurate, precise and RP-HPLC stability-indicating method was developed and validated for the quantitative...

-
Analytical quality by design approach to RP-HPLC method development and validation for simultaneous estimation of esomeprazole and naproxen in modified-release dosage form
BackgroundThe present work describes the development and validation of a new, specific, accurate, and precise stability-indicating RP-HPLC method for...

-
An effective stability indicating RP-HPLC method for simultaneous estimation of Dolutegravir and Lamivudine in bulk and their tablet dosage form
BackgroundA Simple, sensitive, and specific stability indicating reverse phase HPLC method was developed for simultaneous estimation of Lamivudine...

-
Development of novel gradient RP-HPLC method for separation of dapagliflozin and its process-related impurities: insight into stability profile and degradation pathway, identification of degradants using LCMS
BackgroundIn the dapagliflozin (DPF) synthesis, 5-Bromo-2-chlorobenzoic acid (5-BC impurity) and 4-Bromo-1-chloro-2-(4-ethoxybenzyl) benzene (4-BC...

