Search
Search Results
-
Lecanemab: First Approval
Lecanemab (lecanemab-irmb; LEQEMBI™) is a humanized immunoglobulin gamma 1 (IgG1) against aggregated soluble and insoluble forms of amyloid-β...

-
Sotatercept: First Approval
Sotatercept (sotatercept-csrk; WINREVAIR TM ) is an activin signalling inhibitor that is being developed by Merck and Co., Inc. (Rahway, NJ, USA) for...

-
Ublituximab: First Approval
Ublituximab (ublituximab-xiiy; BRIUMVI ™ ) is a glycoengineered anti-CD20 monoclonal antibody developed by TG Therapeutics, Inc. for the treatment of...

-
Danicopan: First Approval
Danicopan (Voydeya ® ) is an oral complement factor D inhibitor that is being developed by Alexion AstraZeneca Rare Disease as add-on treatment to...
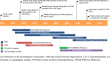
-
Ensitrelvir Fumaric Acid: First Approval
Ensitrelvir fumaric acid (Xocova ® ) is an oral SARS-CoV-2 main protease inhibitor developed by Shionogi for the treatment of SARS-CoV-2 infection. It...
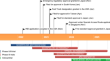
-
Nedosiran: First Approval
Nedosiran (RIVFLOZA™), a once-monthly subcutaneous small interfering RNA (siRNA) therapy, is being developed by Dicerna Pharmaceuticals, a Novo...

-
Zuranolone: First Approval
Zuranolone (ZURZUVAE ™ ) is an oral neuroactive steroid and a positive allosteric modulator of the gamma aminobutyric acid A (GABA A ) receptor being...

-
Resmetirom: First Approval
Resmetirom (Rezdiffra™) is an oral thyroid hormone receptor-β (THR-β) agonist being developed by Madrigal Pharmaceuticals, Inc., to target the key...

-
Talquetamab: First Approval
Talquetamab (talquetamab-tgvs; TALVEY ® ), a humanized, bispecific G-protein coupled receptor family C group 5 member D (GPRC5D)-directed CD3 T-cell...

-
Nadofaragene Firadenovec: First Approval
Nadofaragene firadenovec (nadofaragene firadenovec-vncg; Adstiladrin ® ) is a non-replicating adenoviral vector-based gene therapy developed by Ferring...

-
Pirtobrutinib: First Approval
Pirtobrutinib (Jaypirca TM ), a highly selective, non-covalent, reversible Bruton’s tyrosine kinase (BTK) inhibitor, is being developed by Eli Lilly...

-
Alirocumab: Pediatric First Approval
Alirocumab (Praluent ® ), a proprotein convertase subtilisin kexin type 9 (PCSK9) inhibitor that has been co-developed by Regeneron Pharmaceuticals,...

-
Fezolinetant: First Approval
Fezolinetant (VEOZAH™) is an oral, small molecule, neurokinin 3 receptor (NK3R) antagonist, which is being developed by Astellas Pharma Inc. for the...

-
Retifanlimab: First Approval
Retifanlimab (retifanlimab-dlwr; ZYNYZ TM ) is a programmed cell death 1 receptor-blocking antibody that is being developed by Incyte Corporation for...

-
Avacincaptad Pegol: First Approval
Avacincaptad pegol (IZERVAY™; formerly Zimura ® ) is a complement C5 inhibitor that is being developed by IVERIC Bio, an Astellas company, for the...

-
Fidanacogene Elaparvovec: First Approval
Fidanacogene elaparvovec ( Pr BEQVEZ™) is an adeno-associated viral (AAV) vector-based gene therapy developed by Spark Therapeutics (a subsidiary of...

-
Etrasimod: First Approval
Etrasimod (VELSIPITY™) is an orally available, small-molecule selective sphingosine-1-phosphate (S1P) receptor modulator being developed by Pfizer...

-
Crovalimab: First Approval
Crovalimab (派圣凯 ®; PiaSky) is a humanized, anti-complement component C5 (anti-C5) recycling monoclonal antibody developed by Chugai Pharmaceutical, in...

-
Rezafungin: First Approval
Rezafungin (Rezzayo™), an intravenous once-weekly echinocandin that inhibits 1,3-β- d -glucan synthase, is being developed by Cidara Therapeutics. In...

-
Epcoritamab: First Approval
Epcoritamab (epcoritamab-bysp; Epkinly™; Tepkinly ® ) is a subcutaneously administered CD3×CD20 T-cell-engaging bispecific antibody being co-developed...

