Search
Search Results
-
Role of Artificial Intelligence in Pharmacovigilance
Pharmacovigilance ensures the safety and efficacy of any pharmaceutical drug products or medical devices throughout their lifecycle. Traditional...
-
Adverse drug reactions to chloroquine/hydroxychloroquine in combination with azithromycin in COVID-19 in-patients: data from intensive pharmacovigilance in Morocco, 2020
In Morocco, chloroquine/hydroxychloroquine + azithromycin have been used off-label for COVID-19 treatment. This study aimed to describe the...
-
Pharmacovigilance Through Phased Clinical Trials, Post-Marketing Surveillance and Ongoing Life Cycle Safety
The evolution of science has gifted humans with safe and effective treatment modalities. Drugs are monitored from clinical trials to real world...
-
Pharmacovigilance in the Caribbean Countries: an Overview
Purpose of overviewThe constant surge in accessing essential medicines creates a greater need for continuous monitoring of usage. The inability to...
-
Pharmacovigilance
‘Every drug is a poison’ is a starting line in a toxicology course for pharmacy students. And, there is no drug without a side effect. While side...
-
An Industry Survey on Managing the Pharmacovigilance System Master File in a Global Environment: The Need for a Pragmatic Approach
BackgroundLegislative requirements for Marketing Authorisation Holders (MAHs) to maintain a Pharmacovigilance System Master File (PSMF) were...

-
Pharmacovigilance of Herbal Medicine: An Evolving Discipline
With increase in popularity and use of herbal products both as medicine and nutraceuticals throughout the world, there is an urgent need to monitor...
-
Dispositions and Causality Assessment in Pharmacovigilance: Proposing the Dx3 Approach for Assessing Causality with Small Data Sets
A new approach is proposed for assessing causality in pharmacovigilance. The Dx3 approach is designed to qualitatively evaluate three types of...

-
Specialty Pharmacy Services: ADR Reporting and Pharmacovigilance, Pediatrics, Geriatrics, Women During Pregnancy, Cancer, Pain Management, and Nutrition
Pharmacy comprises of a variety of molecules with potential of action on the living body. The optimization of the use of medicines needs careful...
-
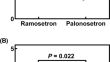
Concomitant palonosetron ameliorates cisplatin-induced nephrotoxicity, nausea, and vomiting: a retrospective cohort study and pharmacovigilance analysis
BackgroundCisplatin (CDDP)-induced nephrotoxicity is the most important complication of CDDP treatment. 5-Hydroxytryptamine type 3 receptor...
-
Automation Opportunities in Pharmacovigilance: An Industry Survey
BackgroundTransCelerate’s Intelligent Automation Opportunities (IAO) in Pharmacovigilance initiative has been working to evaluate various...

-
General Principles of Pharmacovigilance in Clinical Development
Pharmacovigilance is a broad field that spans across all stages of the life cycle from preclinical drug development, clinical development, marketing...
-
The Epidemic of Substandard and Falsified Medications in Iraq: Evaluating the Effectiveness of National Pharmacovigilance Alerts to Community Pharmacies
BackgroundAssessing Iraqi experience with the impact of substandard and falsified (S/F) medicines can help other countries deal comprehensively with...

-
Adverse Events in Pregnant Patients Treated with Coronavirus Disease 2019 Therapeutics
BackgroundPregnant patients are at high risk of maternal and fetal complications from Coronavirus Disease 2019 (COVID-19) infections. The COVID-19...
-
Practical Considerations for the Implementation and Monitoring of Risk Minimisation Measures for High-Risk Teratogenic Medicines
There is considerable societal interest in making medicines more affordable. A critical factor often inadequately considered early in the process of...

-
Role of Artificial Intelligence in Clinical and Hospital Pharmacy
Artificial intelligence (AI) has emerged as an influential force in various industries, with potentially revolutionary implications for healthcare....
-
Serotonin syndrome by drug interactions with linezolid: clues from pharmacovigilance-pharmacokinetic/pharmacodynamic analysis
PurposeTo characterize the post-marketing reporting of serotonin syndrome (SS) due to drug-drug interactions (DDIs) with linezolid and investigate...

-
Job and Career Opportunities in the Pharmaceutical Industry: An Overview
The pharmaceutical industry offers a wealth of job and career opportunities for talented graduates and (young) professionals in the life sciences,...
-
Evolution of adverse drug reactions reporting systems: paper based to software based
ObjectiveAdverse Drug Reactions (ADR) add a significant clinical and economic burden to the healthcare system of a country. We present an overview of...
-
Healthcare Education and Training of Health Personnel
Medicines are an important component of health systems but are often not used rationally. Irrational use can have serious consequences, namely...
