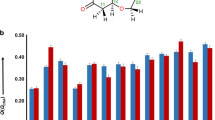
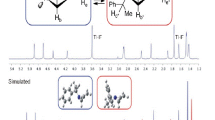
Abstract
Various NMR techniques have been developed that allow assignment of the relative and absolute configuration of many of the stereogenic carbons that occur in marine natural products. The application of chiral anisotropic reagents in conjunction with NMR analyses has been particularly useful for determining the absolute configuration of secondary alcohols, α-substituted primary amines, and α-substituted carboxylic acids. Derivatization of these functional groups with appropriate chiral reagents (e.g., MTPA) provides diastereomeric products that have diagnostic differences in their 1H chemical shifts. A recently developed technique known as J-based configurational analysis uses proton–proton couplings and 2- and 3-bond carbon–proton couplings (2,3 J CH) to assign the relative configuration of adjacent (1, 2) stereogenic carbons in conformationally flexible molecules. The J-based method involves comparing experimentally measured scalar couplings and NOE interactions with those predicted from this model to assign the relative configuration of the chiral methines. This technique is also applicable to oxygenated systems since there is a dihedral angle dependence for 2 J CH couplings between a proton and an adjacent carbon that bears an electronegative oxygen substituent. Strategies have also been developed to utilize J-based configurational analysis when there is a methylene separating the two stereogenic methine carbons, and even when conformational interconversion results in the coexistence of two major conformers.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Dale JA, Mosher HS (1973) Nuclear magnetic resonance enantiomer reagents. Configurations via nuclear magnetic resonance chemical shifts of diastereomeric mandelate, O-methylmandelate, and α-methoxy-α-trifluoromethyphenylacetate (TPA) esters. J Am Chem Soc 95:512–519
Sullivan GR, Dale JA, Mosher HS (1973) Correlation of configuration and 19 F chemical shifts of α-methoxy-α-trifluoromethyphenylacetate derivatives. J Org Chem 38:2143–2147
Ohtani I, Kusumi T, Kashman Y, Kakisawa H (1991) High-field FT NMR applications of Mosher’s method. The absolute configurations of marine terpenoids. J Am Chem Soc 113:4092–4096
Kusumi T, Ohtani II (1999) Determination of the absolute configuration of biologically active compounds by the modified Mosher’s method. In: Cooper R, Snyder JK (eds) The biology chemistry interface. Marcel Dekker, New York, pp 103–137
Seco JM, Quiñoá E, Riguera R (2000) The assignment of absolute configurations by NMR of arylmethoxyacetate derivates: is this methodology being correctly used? Tetrahedron Asymmetry 11:2781–2791
Seco JM, Quiñoá E, Riguera R (2001) A practical guide for the assignment of the absolute configuration of alcohols, amines and carboxylic acids by NMR. Tetrahedron Asymmetry 12:2915–2925
Seco JM, Quiñoá E, Riguera R (2004) The assignment of absolute configuration by NMR. Chem Rev 104:17–117
Oku N, Takada K, Fuller RW, Wilson JA, Peach ML, Pannell LK, McMahon JB, Gustafson KR (2010) Isolation, structural elucidation, and absolute stereochemistry of enigmazole A, a cytotoxic phosphomacrolide from the Papua New Guinea marine sponge Cinachyrella enigmatica. J Am Chem Soc 132:10278–10285
Seco JM, Latypov S, Quiñoá E, Riguera R (1994) New chirality recognizing reagents for the determination of absolute stereochemistry and enantiomeric purity by NMR. Tetrahedron Lett 35:2921–2924
Kusumi T, Takahashi H, Xu P, Fukushima T, Asakawa Y, Hashimoto T, Kan Y, Inouye Y (1994) New chiral anisotropic reagents, NMR tools to elucidate the absolute configurations of long-chain organic compounds. Tetrahedron Lett 35:4397–4400
Williamson RT, Barrios Sosa AC, Mitra A, Seaton PJ, Weibel DB, Schroeder FC, Meinwald J, Koehn FE (2003) New silyl ether reagents for the absolute stereochemical determination of secondary alcohols. Org Lett 5:1745–1748
Nagai Y, Kusumi T (1995) New chiral anisotropic reagents for determining the absolute configuration of carboxylic acids. Tetrahedron Lett 36:1853–1856
Ferreiro MJ, Latypov SK, Quiñoá E, Riguera R (2000) Assignment of the absolute configuration of α–chiral carboxylic acids by 1H NMR spectroscopy. J Org Chem 65:2658–2666
Matsumori N, Kaneno D, Murata M, Nakamura H, Tachibana K (1999) Stereochemical determination of acyclic structures based on carbon-proton spin-coupling constants. A method of configuration analysis for natural products. J Org Chem 64:866–876
Minch MJ (1994) Orientational dependence of vicinal proton-proton NMR coupling constants: the Karplus relationship. Concepts Magn Reson 6:41–56
Bifulco G, Dambruoso P, Gomez-Paloma L, Riccio R (2007) Determination of relative configuration in organic compounds by NMR spectroscopy and computational methods. Chem Rev 107:3744–3779
Nilewski C, Geisser RW, Ebert M-O, Carreira EM (2009) Conformational and configurational analysis in the study and synthesis of chlorinated natural products. J Am Chem Soc 131:15866–15876
Dalvit C, Bovermann G (1995) Pulsed field gradient one-dimensional NMR selective ROE and TOCSY experiments. Magn Reson Chem 33:156–159
Scott K, Keeler J, Van QN, Shaka AJ (1997) One-dimensional NOE experiments using pulsed field gradients. J Magn Reson 125:320–324
Griesinger C, Sørenson OW, Ernst RR (1986) Correlation of connected transitions by two-dimensional NMR spectroscopy. J Chem Phys 85:6837–6852
Márquez B, Gerwick WH, Williamson RT (2001) Survey of NMR experiments for the determination of nJ (C, H) heteronuclear coupling constants in small molecules. Magn Reson Chem 39:499–530
Uhrín D, Batta G, Hruby VJ, Barlow PN, Kövér KE (1998) Sensitivity- and gradient-enhanced hetero (ω1) half-filtered TOCSY experiment for measuring long-range heteronuclear coupling constants. J Magn Reson 130:155–161
Meissner A, Sørenson OW (2001) Measurement of J(HH) and long-range J(X, H) coupling constants in small molecules. Broadband XLOC and J-HMBC. Magn Reson Chem 39:49–52
Williamson RT, Márquez BL, Gerwick WH, Kövér KE (2000) One- and two-dimensional gradient-selective HSQMBC NMR experiments for the efficient analysis of long-range heteronuclear coupling constants. Magn Reson Chem 38:265–273
Bifulco G, Bassarello C, Riccio R, Gomez-Paloma L (2004) Quantum mechanical calculations of NMR J coupling values in the determination of relative configuration in organic compounds. Org Lett 6:1025–1028
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media B.V.
About this entry
Cite this entry
Gustafson, K.R. (2012). NMR Methods for Stereochemical Assignments. In: Fattorusso, E., Gerwick, W., Taglialatela-Scafati, O. (eds) Handbook of Marine Natural Products. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-3834-0_9
Download citation
DOI: https://doi.org/10.1007/978-90-481-3834-0_9
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-90-481-3833-3
Online ISBN: 978-90-481-3834-0
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences