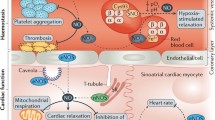
Abstract
Superoxide production plays an important role in the pathophysiological conditions mediated by angiotensin II. It has been shown that inhibition of AT1R inhibits cellular superoxide production and attenuates redox-dependent cell signaling. Animal and cell culture experiments showed that angiotensin II increases superoxide production by NADPH oxidases, uncoupled nitric oxide synthase, xanthine oxidase, and mitochondria. Meanwhile, specific molecular mechanisms of angiotensin II-mediated regulation of superoxide production are not completely understood. In this chapter, we review cellular sources of superoxide and their potential regulations by angiotensin II. Although angiotensin II directly stimulates NADPH oxidases, this results in redox-dependent activation of superoxide production by other cellular sources. It has become clear that cellular sources of superoxide represent a redox-sensitive network which constitutes a feedforward cycle. This mechanism amplifies redox signal under physiological conditions but may represent a vicious cycle of oxidative stress under pathological conditions leading to uncontrolled redox signaling events and cell and tissue dysfunction.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, Iida M (2004) Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation 109(2):227–233
Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87(1):245–313
Bendall JK, Rinze R, Adlam D, Tatham AL, de Bono J, Wilson N, Volpi E, Channon KM (2007) Endothelial Nox2 overexpression potentiates vascular oxidative stress and hemodynamic response to angiotensin II: studies in endothelial-targeted Nox2 transgenic mice. Circ Res 100(7):1016–1025
Boulden BM, Widder JD, Allen JC, Smith DA, Al-Baldawi RN, Harrison DG, Dikalov SI, Jo H, Dudley SC Jr (2006) Early determinants of H2O2-induced endothelial dysfunction. Free Radic Biol Med 41(5):810–817
Cadenas E, Davies KJ (2000) Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 29(3–4):222–230
Cadenas E, Boveris A, Ragan CI, Stoppani AO (1977) Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch Biochem Biophys 180(2):248–257
Cai H, Harrison DG (2000) Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87(10):840–844
Cai H, Li Z, Davis ME, Kanner W, Harrison DG, Dudley SC Jr (2003) Akt-dependent phosphorylation of serine 1179 and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase 1/2 cooperatively mediate activation of the endothelial nitric-oxide synthase by hydrogen peroxide. Mol Pharmacol 63(2):325–331
Chalupsky K, Cai H (2005) Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A 102(25):9056–9061
Chance B, Williams GR (1955) Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem 217(1):409–427
Costa AD, Quinlan CL, Andrukhiv A, West IC, Jaburek M, Garlid KD (2006) The direct physiological effects of mitoK(ATP) opening on heart mitochondria. Am J Physiol Heart Circ Physiol 290(1):H406–H415
DeMarco VG, Habibi J, Whaley-Connell AT, Schneider RI, Heller RL, Bosanquet JP, Hayden MR, Delcour K, Cooper SA, Andresen BT, Sowers JR, Dellsperger KC (2008) Oxidative stress contributes to pulmonary hypertension in the transgenic (mRen2)27 rat. Am J Physiol Heart Circ Physiol 294(6):H2659–H2668
Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK (2008) Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med 45:1340–1351
Dikalova A, Clempus R, Lassegue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HH, Owens GK, Lambeth JD, Griendling KK (2005) Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation 112(17):2668–2676
Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI (2010a) Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 107(1):106–116
Dikalova AE, Gongora MC, Harrison DG, Lambeth JD, Dikalov S, Griendling KK (2010b) Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am J Physiol Heart Circ Physiol 299(3):H673–H679
Doughan AK, Harrison DG, Dikalov SI (2008) Molecular mechanisms of angiotensin II mediated mitochondrial dysfunction. Linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 102(4):488–496
Fridovich I (2004) Mitochondria: are they the seat of senescence? Aging Cell 3(1):13–16
Gorlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, Busse R (2000) A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res 87(1):26–32
Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG (2007) Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204(10):2449–2460
Guzik TJ, Chen W, Gongora MC, Guzik B, Lob HE, Mangalat D, Hoch N, Dikalov S, Rudzinski P, Kapelak B, Sadowski J, Harrison DG (2008) Calcium-dependent NOX5 NADPH oxidase contributes to vascular oxidative stress in human coronary artery disease. J Am Coll Cardiol 52(22):1803–1809
Hamanaka RB, Chandel NS (2010) Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci 35(9):505–513
Han D, Williams E, Cadenas E (2001) Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem J 353(Pt 2):411–416
Hanna IR, Taniyama Y, Szocs K, Rocic P, Griendling KK (2002) NAD(P)H oxidase-derived reactive oxygen species as mediators of angiotensin II signaling. Antioxid Redox Signal 4(6):899–914
Harrison DG, Cai H, Landmesser U, Griendling KK (2003) Interactions of angiotensin II with NAD(P)H oxidase, oxidant stress and cardiovascular disease. J Renin Angiotensin Aldosterone Syst 4(2):51–61
Harrison DG, Chen W, Dikalov S, Li L (2010) Regulation of endothelial cell tetrahydrobiopterin pathophysiological and therapeutic implications. Adv Pharmacol 60:107–132
Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK (2004) Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 24(4):677–683
Hu Z, Chen J, Wei Q, **a Y (2008) Bidirectional actions of hydrogen peroxide on endothelial nitric-oxide synthase phosphorylation and function: co-commitment and interplay of Akt and AMPK. J Biol Chem 283(37):25256–25263
Jones SA, O’Donnell VB, Wood JD, Broughton JP, Hughes EJ, Jones OT (1996) Expression of phagocyte NADPH oxidase components in human endothelial cells. Am J Physiol 271(4 Pt 2):H1626–H1634
Kelley EE, Khoo NK, Hundley NJ, Malik UZ, Freeman BA, Tarpey MM (2010) Hydrogen peroxide is the major oxidant product of xanthine oxidase. Free Radic Biol Med 48(4):493–498
Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, Rahman M, Abe Y (2005a) Mitochondria-derived reactive oxygen species and vascular MAP kinases: comparison of angiotensin II and diazoxide. Hypertension 45(3):438–444
Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, Rahman M, Suzuki T, Maeta H, Abe Y (2005b) Role of NAD(P)H oxidase- and mitochondria-derived reactive oxygen species in cardioprotection of ischemic reperfusion injury by angiotensin II. Hypertension 45(5):860–866
Kuzkaya N, Weissmann N, Harrison DG, Dikalov S (2003) Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem 278(25):22546–22554
Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG (2002) Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension 40(4):511–515
Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG (2003) Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111(8):1201–1209
Landmesser U, Spiekermann S, Preuss C, Sorrentino S, Fischer D, Manes C, Mueller M, Drexler H (2007) Angiotensin II induces endothelial xanthine oxidase activation: role for endothelial dysfunction in patients with coronary disease. Arterioscler Thromb Vasc Biol 27(4):943–948
Lassegue B, Clempus RE (2003) Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 285(2):R277–R297
Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, Griendling KK (2001) Novel gp91(phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res 88(9):888–894
Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG (1997) Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation 95(3):588–593
Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG (2001) Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation 103(9):1282–1288
Lavoie JL, Sigmund CD (2003) Minireview: overview of the renin-angiotensin system – an endocrine and paracrine system. Endocrinology 144(6):2179–2183
Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG (2010) Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55(2):500–507
Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG (2006) Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18(1):69–82
Marvar PJ, Lob H, Vinh A, Zarreen F, Harrison DG (2011) The central nervous system and inflammation in hypertension. Curr Opin Pharmacol 11(2):156–161
McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, Harrison DG (2003) Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol 285(6):H2290–H2297
McNally JS, Saxena A, Cai H, Dikalov S, Harrison DG (2005) Regulation of xanthine oxidoreductase protein expression by hydrogen peroxide and calcium. Arterioscler Thromb Vasc Biol 25(8):1623–1628
Park YM, Lim BH, Touyz RM, Park JB (2008) Expression of NAD(P)H oxidase subunits and their contribution to cardiovascular damage in aldosterone/salt-induced hypertensive rat. J Korean Med Sci 23(6):1039–1045
Queliconi BB, Wojtovich AP, Nadtochiy SM, Kowaltowski AJ, Brookes PS (2011) Redox regulation of the mitochondrial K(ATP) channel in cardioprotection. Biochim Biophys Acta 1813(7):1309–1315
Rasmussen JT, Rasmussen MS, Petersen TE (2000) Cysteines involved in the interconversion between dehydrogenase and oxidase forms of bovine xanthine oxidoreductase. J Dairy Sci 83(3):499–506
Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ (2001) Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(−) and systolic blood pressure in mice. Circ Res 89(5):408–414
Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK (2002) Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res 91(5):406–413
Sun J, Druhan LJ, Zweier JL (2008) Dose dependent effects of reactive oxygen and nitrogen species on the function of neuronal nitric oxide synthase. Arch Biochem Biophys 471(2):126–133
Sun J, Druhan LJ, Zweier JL (2010) Reactive oxygen and nitrogen species regulate inducible nitric oxide synthase function shifting the balance of nitric oxide and superoxide production. Arch Biochem Biophys 494(2):130–137
Takac I, Schröder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, Brandes RP (2011) The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem 286(15):13304–13313
Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, Schiffrin EL (2002) Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res 90(11):1205–1213
Ushio-Fukai M, Griendling KK, Becker PL, Hilenski L, Halleran S, Alexander RW (2001) Epidermal growth factor receptor transactivation by angiotensin II requires reactive oxygen species in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 21(4):489–495
Wang HD, Pagano PJ, Du Y, Cayatte AJ, Quinn MT, Brecher P, Cohen RA (1998) Superoxide anion from the adventitia of the rat thoracic aorta inactivates nitric oxide. Circ Res 82(7):810–818
Wang HD, Johns DG, Xu S, Cohen RA (2002) Role of superoxide anion in regulating pressor and vascular hypertrophic response to angiotensin II. Am J Physiol Heart Circ Physiol 282(5):H1697–H1702
Widder JD, Fraccarollo D, Galuppo P, Hansen JM, Jones DP, Ertl G, Bauersachs J (2009) Attenuation of angiotensin II-induced vascular dysfunction and hypertension by overexpression of thioredoxin 2. Hypertension 54(2):338–344
Yamamoto E, Kataoka K, Yamashita T, Tokutomi Y, Dong YF, Matsuba S, Ogawa H, Kim-Mitsuyama S (2007) Role of xanthine oxidoreductase in the reversal of diastolic heart failure by candesartan in the salt-sensitive hypertensive rat. Hypertension 50(4):657–662
Zuo L, Ushio-Fukai M, Hilenski LL, Alexander RW (2004) Microtubules regulate angiotensin II type 1 receptor and Rac1 localization in caveolae/lipid rafts: role in redox signaling. Arterioscler Thromb Vasc Biol 24(7):1223–1228
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this entry
Cite this entry
Dikalov, S.I., Harrison, D.G. (2014). Angiotensin II and Superoxide Generation. In: Laher, I. (eds) Systems Biology of Free Radicals and Antioxidants. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-30018-9_59
Download citation
DOI: https://doi.org/10.1007/978-3-642-30018-9_59
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-30017-2
Online ISBN: 978-3-642-30018-9
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences