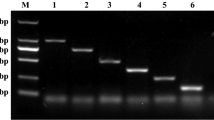
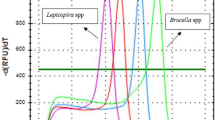
Abstract
Molecular diagnostics have revolutionized the efficiency of diagnosis of infectious diseases. An accurate diagnosis of a disease would prevent further infestation of infections in the healthy population. Although several laboratory techniques exists such as cell-culture, serological, and molecular based methods that were developed in the past decades for highlighting these diseases, however, because of their robustness, high sensitivity, specificity, rapidness, and suitability of the range of types of samples that can be analyzed, nucleic acid amplification tests are suitable for the diagnosis of pathogenic infections. Since the development of polymerase chain reaction (PCR), this molecular technique has been widely utilized as a nucleic acid amplification tool and besides being utilized as a laboratory tool, it has proved to have an exceptional potential in clinical applications, including detection of specific or broad spectrum pathogens, evaluation of emerging novel infections, disease surveillance, early threat of bio-threat agents, and antimicrobial resistance profiling. The multiplex PCR is the technique where we can simultaneously detect more than one organism in a single reaction. The multiplexing may be done either with conventional PCR or with real time PCR. This technique provides detection of mixed infection including both viruses and bacteria, time saving and cost effective.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Karch H, Meyer T (1989) Single primer pair for amplifying segments of distinct Shiga-like toxin genes by polymerase chain reaction. J Clin Microbiol 27(12):2751–2757
Meyer T, Bitzan M, Sandkamp O, Karch H (1989) Synthetic oligodeoxyribonucleotide probes to detect verocytptoxin – producing Escherichia coli in diseased pigs. FEMS Microbiol Lett 57(2):247–252
Belak S, Ballagi-Pordany A, Flensburg J, Virtanen A (1989) Detection of pseudorabies virus DNA sequences by polymerase chain reaction. Arch Virol 108(3–4):279–286
Jestin A, Foulon T, Pertuiset B, Blanchard P, Labourdet M (1990) Rapid detection of pseudorabies virus genome sequences in biological samples from infected pigs using polymerase chain reaction DNA amplification. Vet Microbiol 1(4):317–328
Xufu Y, Faquan P (1990) Confirmation and diagnosis of Porcine Haemophilus pleuropneumonia infection in China. Chinese J Anim Vet Sci
Rasschaert D, Duarte M, Laurde H (1990) Porcine respiratory coronavirus differs from transmissible gastroenteritis virus by a few genomic deletions. J Gen Virol 71(11):2599–2607
Meyer RF, Brown CC, House C, House JA, Molitor TW (1991) Rapid and sensitive detection of foot-and-mouth disease virus in tissues by enzymatic RNA amplification of the polymerase gene. J Virol Methods 34(2):161–172
Molitor TW, Oraveerakul K, Zhang QQ, Choi CS, Ludemann LR (1991) Polymerase chain amplification (PCR) amplification for detection of porcine parvovirus. J Virol Methods 32(2–3):201–211
Gouvea V, Santos N, Timenetsky MDC (1994a) Identification of bovine and porcine rotavirus G types by PCR. J Clin Microbiol 32(5):1338–1340
Gouvea V, Santos N, Timenetsky MDC (1994b) VP4 ty** of bovine and porcine group A rotaviruses by PCR. J Clin Microbiol 32(5):1333–1337
Suarez P, Zardoya R, Prieto C, Solana A, Tabares E, Bautista JM, Castro JM (1994) Direct detection of the porcine reproductive and respiratory syndrome (PRRS) virus reverse polymerase chain reaction (RT-PCR). Arch Virol 135:89–99
Chamberlain JS, Gibbs RA, Ranier JE, Nguyen PN, Caskey CT (1988) Deletion screening of the Duchenne muscular dystrophy locus via multiplex DNA amplification. Nucleic Acids Res 16:11141–11156
La T, Collins AM, Phillips ND, Oksa A, Hampson DJ (2006) Development of multiplex PCR for rapid detection of enteric pathogens Lawsonia intracellularis, Brachyspira hyodysenteriae, and Brachyspira pilosicoli in porcine faeces. Lett Appl Microbiol 42(3):284–288
Ogawa H, Taira O, Hirai T, Takeuchi H, Nagao A, Ishikawa Y, Tuchiya K, Nunoya T, Ueda S (2009) Multiplex PCR and multiplex RT-PCR for inclusive detection of major swine DNA and RNA viruses in pigs with multiple infections. J Virol Methods 160:210–214
Saiki RK (1989) The design and optimization of the PCR. In: PCR technology, principles and applications for DNA amplification. Stockton Press, New York, pp 7–16
Rajkhowa S (2015) Development of a novel multiplex PCR assay for rapid detection of virulence associated genes of Pasteurella multocida from pigs. Lett Appl Microbiol 61(3):293–298
Henegariu O, Heerema NA, Dlouhy SR, Vance GH, Vogt PH (1997) Multiplex PCR: critical parameters and step-by-step protocol. BioTechniques 23:504–511
Zangenberg G, Saiki R, Reynolds R (1999) Multiplex PCR: Optimization Guidelines. In: Chapter 6, PCR applications protocols for functional genomics. Academic Press, pp 73–94
Xu XG, Chen GD, Huang Y, Ding L, Li ZC, Chang CD, Wang CY, Tong DW, Liu HJ (2012) Development of multiplex PCR for simultaneous detection of six swine DNA and RNA viruses. J Virol Methods 183(1):69–74
Apte A, Daniel S (2003) PCR primer design. In: Chapter 7, PCR Primer A laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbour, NY, pp 61–74
Mahony JB, Chernesky MA (1995) Multiplex polymerase chain reaction. In: Chapter 10, Molecular methods for virus detection. Academic Press, San Diego, pp 219–236
Loewy ZG, Mecca J, Diaco R (1994) Enhancement of Borrelia burgdorferi PCR by uracil N- glycosylase. J Clin Microbiol 32(1):135–138
Erlich H, Gelfand D, Sninsky J (1991) Recent advances in the polymerase chain reaction. Science 252:1643–1651
Mahony J, Luinstra K, Sellors J, Chernesky M (1993) Comparison of plasmid and chromosome based polymerase chain reaction assays for detecting Chlamydia trachomatis nucleic acids. J Clin Microbiol 9:2241–2245
Gilbert SA, Larochelle R, Magar R, Cho HJ, Deregt D (1997) Ty** of porcine reproductive and respiratory syndrome viruses by a multiplex PCR assay. J Clin Microbiol 35(1):264–267
Gerilovych A, Stegniy B, Golovko V, Solodiankin O, Gerilovych I, Smolyaninova Y, Rudova N (2015) Development of the multiplex PCR for detection of the DNA contained emergent diseases agents in pigs (African swine fever, Aujeszky disease, Circoviral disease). Online J Public Health Inform 7(1):e131
Yang K, Jiao Z, Zhou D, Guo R, Duan Z, Tian Y (2019) Development of a multiplex PCR to detect and discriminate porcine circoviruses in clinical specimens. BMC Infect Dis 19:778
Chen HY, Wei ZY, Zhang HY, Lu XL, Zheng LI, Cui CA, Liu J, Zhu QL, Wang ZX (2010) Use of multiplex RT-PCR assay for simultaneous detection of North American genotype PRRSV, swine influenza virus and Japanese encephalitis virus. Agric Sci China 9(7):1050–1057
Fujii Y, Doan YH, Wahyuni RM, Lusida MI, Utsumi T, Shoji I, Kazuhiko K (2019) Improvement of rotavirus genoty** method by using the semi-nested multiplex PCR with new primer set. Front Microbiol 10:647
Hu L, Lin XY, Yang ZX, Yao XP, Li GL, Peng SZ, Wang Y (2015) A multiplex PCR for simultaneous detection of classical swine fever virus, African swine fever virus, highly pathogenic porcine reproductive and respiratory syndrome virus, porcine reproductive and respiratory syndrome viruses and pseudorabies in swine. Pol J Vet Sci 18(4):715–723
Phillips ND, La T, Adams PJ, Harland BL, Fenwick SG, Hampson DJ (2009) Detection of Brachyspira hyodysenteriae, Lowsonia intracellularis, and Brachyspira pilosicoli in feral pigs. Vet Microbiol 134(3–4):294–299
Elder RO, Duhamel GE, Mathiesen MR, Erickson ED, Gebhart CJ, Oberst RD (1997) Multiplex polymerase chain reaction for simultaneous detection of Lowsonia intracellularis, Serpulina hyodysenteriae and salmonellae in porcine intestinal specimens. J Vet Diagn Investig 9(3):281–286
Rajkhowa S, Sarma D, Shakuntala I, Sahoo N (2015) Multiplex PCR assay for simultaneous detection of three important pathotypes of Escherichia coli from diarrhoeic piglets. Indian J Anim Sci 85(11):1202–1204
Lung O, Adjei SO, Buchanan C, Joseph T, King R, Erickson A, Detmer S, Ambagala A (2017) Multiplex PCR and microarray for detection of swine respiratory pathogens. Transbound Emerg Dis 64(3):834–848
Kim J, Chae C (2003) Multiplex nested PCR compared with insitu hybridization for the differentiation of porcine circoviruses and porcine parvovirus from pigs with postweaning multisystemic wasting syndrome. Can J Vet Res 67(2):133–137
Pan QX, Chen D, He KW, Huang KH (2005) Multiplex PCR for rapid detection of pseudorabies virus, PPV and PCV-2. Virol Sin 20(6):603–606
Meer RR, Songer JG (1997) Multiplex polymerase chain reaction assay for genoty** Clostridium perferingens. Am J Vet Res 58(7):702–705
Kanakaraj R, Harris DL, Songer JG, Bosworth B (1998) Multiplex PCR assay for detection of Clostridium perferingens in feces and intestinal contents of pigs and in swine feed. Vet Microbiol 63(1):29–38
Seungtae K, Sharma N, Seog KH, Young MG, Kyoo OS, Yi PS, Yeun LJ, Jeong DK (2014) Establishment of diagnostic test for enteric diarrhea in pigs using efficient multiplex polymerase chain reaction. Indian J Anim Sci 84(11):1157–1162
Liu G, Jiang Y, Opriessnig T, Gu K, Zhang H, Yang Z (2019) Detection and differentiation of five diarrhea related pig viruses utilizing a multiplex PCR assay. J Virol Methods 263:32–37
Ding G, Fu Y, Li B, Chen J, Wang J, Yin B, Sha W, Liu G (2019) Development of a multiplex RT-PCR for the detection of major diarrhoeal viruses in pig herds in China. Transbound Emerg Dis 67(2):678–685
Beck R, Beck A, Lucinger S, Florijancic T, Boskovic I, Marinculic A (2009) Vet Parasitol 159(3–4):304–307
Lin RQ, Ai L, Zou FC, Verweij JJ, Jiang Q, Li MW, Song HQ, Zhu XQ (2008) A multiplex PCR tool for the specific identification of Oesophagostomum spp. from pigs. Parasitol Res 103(4):993–997
Sato MO, Cavalcante TV, Sako Y, Nakao M, Yamasaki H, Yatsuda AP, Nakaya K, Ito A (2006) Evidence and potential for transmission of human and swine Taenia solium Cysticercosis in the Piracuruca region Piaui, Brazil. Am J Trop Med Hyg 75(5):933–935
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Choudhury, M., Yadav, A.K., Pegu, S.R., Deb, R., Rajkhowa, S. (2022). Multiplex PCR for Diagnosis of Porcine Diseases. In: Deb, R., Yadav, A.K., Rajkhowa, S., Malik, Y.S. (eds) Protocols for the Diagnosis of Pig Viral Diseases. Springer Protocols Handbooks. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2043-4_5
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2043-4_5
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2042-7
Online ISBN: 978-1-0716-2043-4
eBook Packages: Springer Protocols