Abstract
Astrocyte reactivity and neuroinflammation are hallmarks of CNS pathological conditions such as Alzheimer’s disease. However, the specific role of reactive astrocytes is still debated. This controversy may stem from the fact that most strategies used to modulate astrocyte reactivity and explore its contribution to disease outcomes have only limited specificity. Moreover, reactive astrocytes are now emerging as heterogeneous cells and all types of astrocyte reactivity may not be controlled efficiently by such strategies.
Here, we used cell type-specific approaches in vivo and identified the JAK2-STAT3 pathway, as necessary and sufficient for the induction and maintenance of astrocyte reactivity. Modulation of this cascade by viral gene transfer in mouse astrocytes efficiently controlled several morphological and molecular features of reactivity. Inhibition of this pathway in mouse models of Alzheimer’s disease improved three key pathological hallmarks by reducing amyloid deposition, improving spatial learning and restoring synaptic deficits.
In conclusion, the JAK2-STAT3 cascade operates as a master regulator of astrocyte reactivity in vivo. Its inhibition offers new therapeutic opportunities for Alzheimer’s disease.
Similar content being viewed by others
Introduction
Astrocytes become reactive in virtually all diseases of the central nervous system (CNS). Astrocyte reactivity is classically defined by two hallmarks: cellular hypertrophy and overexpression of intermediate filament proteins such as Glial Fibrillary Acidic Protein (GFAP) and vimentin [27]. But astrocyte reactivity involves significant transcriptional changes that go well beyond these two morphological alterations, and the functional consequences of reactivity are unclear [7, 9, 47, 67]. In Alzheimer’s disease (AD), the most frequent neurodegenerative disease (ND) characterized by amyloid and Tau deposition in the brain, as well as memory loss and synaptic alterations, the role of reactive astrocytes is still debated [13, 63].
Studies in cellular or mouse models of AD report changes in specific astrocyte functions, including metabolic support [2, 72], neurotransmitter recycling [51], antioxidant defense [2, 83] and synaptic transmission [31, 45]. However, the overall contribution of reactive astrocytes to AD remains unclear, because the changes reported in these studies can have beneficial, detrimental or mixed effects on neurons. Attempts to block astrocyte reactivity globally, through knock-out of astrocyte intermediate filament proteins [32, 5: Table S2), emphasizing that markers of these two extreme classes are co-regulated by SOCS3. This module also contained genes related to inflammation (e.g. C1qa, C1qb, C1qc, Ccl3, Additional file 5: Table S2, Additional file 6: Figure S4). We performed an interaction network analysis with STRING on the 100 most connected genes of the module. Gene networks linked to complement system/inflammation were identified, as well as cytoskeleton and cell adhesion, which may underlie the morphological changes characteristic of reactive astrocytes (Additional file 6: Figure S4).
SOCS3 inhibits the expression of reactive astrocyte markers. a, Heatmaps of genes belonging to the pan, A1 or A2 reactive astrocyte cassettes. SOCS3 decreases the expression of markers belonging to all categories, in APP astrocytes. Color scales represent mean-centered expression (log2-transformed). Wald test. b, Dendrogram obtained by WGCNA with the significant module indicated with an arrow. c, The significant WGCNA module is mainly formed by genes down-regulated by SOCS3. ANOVA. N = 7-4-5. * p < 0.05, ** p < 0.01, *** p < 0.001
Overall, these results show that SOCS3 operates as a master inhibitor on transcriptional programs of reactivity, regulating different neuroinflammatory markers in reactive astrocytes.
JAK2-STAT3-mediated astrocyte reactivity promotes amyloid deposition in APP mice
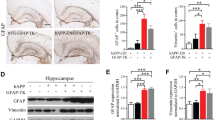
The identification of a master regulator of reactive astrocytes makes it possible to evaluate their overall contribution to AD pathological outcomes. We first investigated the impact of JAK2-STAT3-mediated astrocyte reactivity on amyloid deposition, a major histopathological hallmark of AD [24]. SOCS3-mediated inhibition of astrocyte reactivity significantly reduced the number of BAM10+ amyloid plaques in the hippocampus of 9 month-old APP mice (Fig. 4a, b). This effect was also observed after labeling plaques with methoxy-XO4 (MXO4), a fluorescent Congo red-derivative that binds aggregated amyloid (− 36%, p < 0.05, Student t test, data not shown). Importantly, over-activation of astrocytes with JAK2ca had opposite effects (Fig. 4a, b). The average size of individual plaques was identical between groups, suggesting that only the number, not the properties of plaques was impacted by SOCS3 (Fig. 4c). Levels of soluble human amyloid β (Aβ)42 and Aβ40 peptides, and their ratio were not significantly different between APP-GFP, APP-SOCS3 or APP-JAK2ca mice (Fig. 4d). Moreover, changes in amyloid plaque load were not due to changes in the expression of proteins involved in amyloid precursor protein (APP) metabolism. Indeed, protein levels of APP itself, of the pro-amyloïdogenic β-secretase BACE1, or of insulin degrading enzyme (IDE) and apolipoprotein E (ApoE), two proteins released by astrocytes and involved in Aβ elimination, were not impacted by SOCS3 or JAK2ca (Additional file 7: Figure S5).
Inhibition of JAK2-STAT3-mediated astrocyte reactivity reduces amyloid load in APP mice without impacting microglial cells a, Representative images of BAM10+ amyloid plaques (white) automatically delineated in yellow in APP-GFP, APP-SOCS3 and APP-JAK2ca mice. b, The number of hippocampal BAM10+ plaques is significantly decreased by SOCS3 (N = 9–8) and increased by JAK2ca in APP mice (N = 6–6). SOCS3 and JAK2ca effects were measured in two independent cohorts. c, The average size of individual BAM10+ amyloid plaque is similar between groups. d, Dosage of Aβ40 and Aβ42 peptide concentrations in Triton-X100-soluble protein homogenates from the hippocampus of APP-GFP (N = 10 or 6), APP-SOCS3 (N = 8) and APP-JAK2ca mice (N = 6). Aβ40 and 42 levels are not significantly different between groups. e, IBA1+ microglial cells (red, arrowheads) in contact with a MXO4+ amyloid plaque (blue). f, The number of microglia per plaque is similar between APP-GFP and APP-SOCS3 mice. N = 10–8. g, Confocal images of MXO4+ material (blue) in IBA1+ microglial cells (red). Microglial cells in contact with plaques either display MXO4 staining (white arrowhead) at the membrane, in the cytosol or are MXO4−. h, The proportion of these three classes of microglial cells is not different between groups. N = 10–8. i, Experimental design to monitor Aβ phagocytosis. WT-GFP (N = 10), APP-GFP (N = 6) and APP-SOCS3 (N = 8) mice were injected with MXO4, 3 h before sacrifice. After staining, hippocampal CD11b+/CD45+ microglia and GFP+ astrocytes were analyzed by FACS. j, Representative gates to analyze MXO4+ amyloid uptake in astrocytes and microglia. There are 20% MXO4+ microglial cells in both APP-GFP and APP-SOCS3 groups and no MXO4+ astrocytes. k, No difference in the MXO4 median fluorescent intensity (MFI) is observed between APP-GFP and APP-SOCS3 microglial cells. l-m, RT-qPCR analysis on microglial cells acutely isolated from the hippocampus of 12 month-old WT-GFP, APP-GFP and APP-SOCS3 mice. l mRNA levels of Ctss and C1qb, two microglial homeostatic genes, is similar in all groups. m, Apoe and Trem2 mRNA levels are higher in phagocytic MXO4+ microglia than non-phagocytic MXO4− microglia, while Tmem119 levels are lower in MXO4+ microglia. This transcriptional profile is reminiscent of DAM microglia [34]. Astrocyte de-activation by SOCS3 does not impact the transcriptional profile of either type of microglia. N = 3–8/group. b, d, h, Student t test. c, f, k, Kruskall-Wallis test. l, m, One way ANOVA to compare the 3 groups within MXO4− cells and Student t test to compare two groups within MXO4+ cells. Mann-Whitney test to compare MXO4+ and MXO4− microglial cells within APP-GFP or APP-SOCS3 groups. * p < 0.05, ** p < 0.01
We then focused on SOCS3, as its plaque lowering effects were more therapeutically relevant. Because microglia play a key role in amyloid plaque elimination via phagocytosis [42], we investigated whether inhibition of astrocyte reactivity increases microglia phagocytic activity, which could explain decreased amyloid load in APP-SOCS3 mice. The number of IBA1+ microglial cells in contact with MXO4+ amyloid plaques was not different between APP-SOCS3 and APP-GFP mice (Fig. 4e, f). MXO4 also labelled intracellular amyloid material in IBA1+ microglial cells on brain sections. In fact, more than 95% of microglia cells in contact with plaques were MXO4+, revealing active microglial phagocytosis of amyloid plaques (Fig. 4g, h). MXO4+ material was either localized at the membrane or within the soma (Fig. 4g), but the relative localization of MXO4+ within microglia was not different between APP-SOCS3 and APP-GFP mice (Fig. 4h). To have dynamic quantification of microglia (and astrocyte) phagocytic capacity, MXO4 was injected i.p. 3 h before mouse sacrifice and its accumulation within dissociated brain cells was monitored by FACS. Astrocytes were identified by their GFP expression, while microglial cells were labelled with CD11b and CD45 antibodies (Fig. 4i). The percentages of GFP+ astrocytes (4%) and total microglial cells (14%) were not different between WT-GFP, APP-GFP and APP-SOCS3 mice (data not shown). No MXO4 uptake was detected in WT cells (Fig. 4j). Strikingly, we found no evidence for active MXO4 uptake in GFP+ astrocytes (Fig. 4j). In addition, the expression of several receptors or proteins involved in phagocytosis (Abca1, Apoe, Axl, Gulp1, Itgav, Itgb5, Ldlr, Lrp1, Megf10, Mertk, [10, 55]) were expressed at similar levels in astrocytes of the three groups or only down-regulated by SOCS3 (Fcer1g, Fcgr2b and Fcgr3, Additional file 8: Table S3), further suggesting that astrocyte phagocytosis is not involved in reduced amyloid deposition with SOCS3. In contrast to astrocytes, ~ 20% of CD11b+/CD45+ microglial cells accumulated MXO4+ material in both APP-GFP and APP-SOCS3 mice (Fig. 4j). MXO4 median fluorescent intensity in microglia was not different between these two groups (Fig. 4k). A sub-class of microglia with active phagocytic capacities was recently described as « disease-associated microglia » DAM, [34]. It is possible that inhibition of astrocyte reactivity with SOCS3 only impacts a specific class of microglial cells like DAM. To explore this possibility, expression of genes specific for homeostatic microglia (Ctss and C1qb) and DAM cells were analyzed in MXO4− and MXO4+ microglial cells. MXO4+ microglia had a gene profile characteristic of DAM (up-regulation of ApoE and Trem2; downregulation of Tmem119), consistent with the strong phagocytic activity of these cells. SOCS3 expression in astrocytes did not change the levels of homeostatic microglia or DAM-specific markers (Fig. 4l, m).
Overall, we show that SOCS3 does not significantly impact microglial molecular profile or phagocytic capacity. The reduction in amyloid deposition with SOCS3 is not due to enhanced phagocytosis by glial cells.
Inhibition of astrocyte reactivity improves spatial learning in APP mice
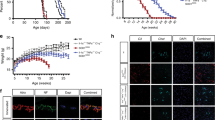
We next tested whether SOCS3-mediated inhibition of astrocyte reactivity improved behavioral defects in APP mice. Nine month-old WT-GFP, APP-GFP and APP-SOCS3 mice were tested on the Morris water maze to assess spatial learning and memory. Swim speed was not different between groups (p = 0.63 for group effect, repeated-measure ANOVA). As previously described [16], APP-GFP mice displayed delayed task learning, as they only improved their ability to find the hidden platform by training day 4, while WT-GFP mice achieved this performance by day 2 (Fig. 5a, repeated-measures ANOVA p = 0.0136 for group effect, p < 10− 6 for day effect). By contrast, APP-SOCS3 mice learnt the task as quickly as WT-GFP mice, showing that SOCS3 corrects learning deficits in APP mice. Mice were probed for spatial memory 72 h after the last day of training. WT-GFP mice displayed the expected preference for the target quadrant, while APP-GFP mice explored all quadrants similarly (Fig. 5b). SOCS3 did not improve spatial memory in APP mice (Fig. 5b), showing that inhibition of hippocampal astrocyte reactivity by SOCS3 improves spatial learning but not memory retrieval in APP mice.
Inhibition of STAT3-mediated astrocyte reactivity improves spatial learning in APP mice a, Training phase of the Morris water maze. APP-GFP mice (N = 12) need more trials to learn the task than WT-GFP mice (N = 11). This learning deficit is corrected by SOCS3 expression in APP astrocytes (N = 11). Repeated-measures ANOVA. b, Probe phase of the Morris Water maze, 72 h after the last training session. Unlike WT-GFP mice, APP-GFP mice do not display preference for the target quadrant (T) over other quadrants (O). This memory deficit is not corrected by SOCS3. Wilcoxon test. * p < 0.05
SOCS3 restores synaptic transmission and plasticity in 3xTg mice
Given that astrocyte reactivity inhibition restores a complex behavioral task, we then examined the underlying cellular mechanisms in acute hippocampal slices. We studied synaptic transmission and plasticity, which are altered in AD [74] and regulated by astrocytes [5]. For this study, we used 3xTg-AD mice (thereafter called 3xTg mice) that display more robust synaptic deficits than APP mice [50]. 3xTg mice show early impairment in synaptic transmission and long-term plasticity before amyloid and Tau pathology [59]. 3xTg mice were injected at 3–4 months with AAV-GFP or AAV-SOCS3 + AAV-GFP (3xTg-GFP and 3xTg-SOCS3 mice respectively) and studied 5 months later, when they display synaptic deficits and higher GFAP levels [11, 53] but before amyloid plaque and Tau deposition ([59], Fig. 6a). Like in APP mice, SOCS3 was able to block astrocyte reactivity in the hippocampus of 3xTg mice, as seen by significantly lower GFAP levels in 3xTg-SOCS3 mice than in 3xTg-GFP mice (Fig. 6b). We then studied basal glutamatergic synaptic transmission, short-term and long-term synaptic plasticity in acute hippocampal slices prepared from the three groups. Field excitatory post-synaptic potentials (fEPSPs) were recorded in the infected, GFP+ region of the stratum radiatum in the CA1 area (Fig. 6a). As reported previously in 3xTg-GFP mice [59], the input-output relationship for evoked fEPSPs at CA3-CA1 synapses was shifted to the right, indicating impaired basal glutamatergic synaptic transmission (Fig. 6c). This was probably not due to a modification of glutamate release probability because the paired-pulse ratio was unchanged (Fig. 6c, d). Interestingly, inhibition of astrocyte reactivity by SOCS3 rescued the input-output relationship (Fig. 6c). As reported before [59], LTP induced by high frequency stimulation (HFS) of Schaffer collaterals was impaired in 3xTg-GFP mice, in comparison to WT-GFP mice that displayed a 50% increase in fEPSPs after HFS (Fig. 6e, f). Strikingly, SOCS3 fully restored LTP deficits in 3xTg mice (Fig. 6e, f), suggesting that astrocyte reactivity plays a major role in AD-related synaptic alterations, and that these alterations can be reversed.
SOCS3 rescues synaptic transmission and long-term plasticity in the hippocampus of 3xTg mice. a, Acute hippocampal slices were prepared from the hippocampus of 8–9 month-old WT-GFP, 3xTg-GFP and 3xTg-SOCS3 mice. A recording electrode was placed in the stratum radiatum of the GFP+ CA1 region. b, Acute slices processed for GFAP immunohistochemistry (red). In 3xTg-GFP mice, astrocytes display higher GFAP immunoreactivity and tortuous processes, compared to WT-GFP controls. SOCS3 restores low GFAP levels in 3xTg astrocytes N = 8–7-6. c, Representative traces for WT-GFP, 3xTg-GFP and 3xTg-SOCS3 mice after a paired-pulse stimulation protocol (50 ms interval) with increasing voltage. The input/output relationship is impaired in 3xTg-GFP mice and restored by SOCS3. N = 11-7-7. Two way (group, voltage) ANOVA and Tukey’s test. d, The paired-pulse ratio (PPR) at 50 V is similar in the three groups. N = 11-7-7. ANOVA. e, Representative (left) and average (right) fEPSPs before (1) and after (2) HFS protocol in the three groups. LTP is impaired in 3xTg mice and restored by SOCS3. f, Normalized fEPSP slopes 40 to 50 min post HFS, relatively to fEPSPs measured 10 min before HFS. N = 6-6-5. ANOVA and Tukey’s test. * p < 0.05, ** p < 0.01, *** p < 0.001
JAK2-STAT3 pathway activation in astrocytes induces reactivity and synaptic alterations
We reasoned that if synaptic deficits in 3xTg mice are due to JAK2-STAT3 pathway activation in astrocytes, its stimulation in naïve WT mice should result in comparable synaptic deficits.
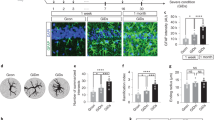
To this end, we first studied whether activation of the JAK2-STAT3 pathway by JAK2ca was sufficient to induce astrocyte reactivity in the hippocampus of WT mice (Fig. 7a). As expected, we found that JAK2ca significantly increased STAT3 immunoreactivity in hippocampal astrocytes (Fig. 7b, c). Importantly, immunoreactivity for GFAP was increased in a large part of the hippocampus of WT-JAK2ca mice (Fig. 7d-f). JAK2ca-astrocytes also overexpressed vimentin (Fig. 7e) and had enlarged soma with tortuous processes (Fig. 7e, g). RT-qPCR analysis on acutely isolated astrocytes from WT-JAK2ca or WT-GFP mice confirmed that JAK2ca significantly increased mRNA levels of reactive genes (Gfap, serpina3n) in astrocytes (Fig. 7h). Overall, activation of the JAK2-STAT3 pathway in astrocytes was sufficient to induce morphological and molecular hallmarks of reactivity in the complete absence of pathological environment.
JAK2ca activates the JAK2-STAT3 pathway and induces astrocyte reactivity. a, WT mice were injected in the hippocampus with AAV-GFP alone (N = 6) or AAV-JAK2ca + AAV-GFP (JAK2ca + GFP, N = 6) at the same total viral titer and were studied 1–2 months later. b, Confocal images of hippocampal sections, stained for GFP (green) and STAT3 (cyan) in WT-GFP or WT-JAK2ca mice. JAK2ca induces STAT3 upregulation and nuclear accumulation in astrocytes, indicating STAT3 activation (arrowhead). c, STAT3 IR quantification in astrocyte nucleus from images in b. d, Representative low magnification images showing the transduced area (GFP+, green) and corresponding GFAP staining (magenta) in the hippocampus of WT-GFP and WT-JAK2ca mice. JAK2ca increases GFAP levels in a large part of the hippocampus (outlined). e, Confocal images of astrocytes stained for GFP (green), GFAP (magenta) and vimentin (red) in WT-GFP and WT-JAK2ca mice. JAK2ca increases GFAP and vimentin expression in hippocampal astrocytes and induces morphological changes. f, GFAP IR is increased by 70% in JAK2ca-injected hippocampus. g, Sholl analysis applied to GFAP-labelled astrocytes shows that reactive astrocytes in WT-JAK2ca mice have a larger domain area and a higher ramification index, a measure of cell complexity. h, RT-qPCR analysis was performed on acutely sorted hippocampal astrocytes from WT-GFP and WT-JAK2ca mice. Jak2, Gfap and Serpina3n are significantly overexpressed in WT-JAK2ca astrocytes. N = 7/group. c, f, g, Student t test. h, Gfap, Serpina3n: Student t test, Jak2: Mann-Whitney test. * p < 0.05, ** p < 0.01, *** p < 0.001
We then recorded synaptic activity in the infected GFP+ hippocampal CA1 region of WT-GFP and WT-JAK2ca mice (Fig. 8a). JAK2ca-mediated astrocyte reactivity impaired basal glutamatergic synaptic transmission (Fig. 8b) but it did not impact the paired-pulse ratio (Fig. 8c). Strikingly, HFS protocol failed to induce LTP in JAK2ca mice, as in 3xTg mice, while it induced a 60% increase in fEPSP in WT-GFP mice (Fig. 8d, e), showing that JAK2ca causes significant deficits in long-term synaptic plasticity. Taken together, our results show that JAK2-STAT3-mediated astrocyte reactivity induces synaptic dysfunction in the hippocampus, and is a potent target for synaptic restoration in AD.
JAK2ca-induced astrocyte reactivity is sufficient to alter synaptic transmission and long-term plasticity in WT mice. a, Acute hippocampal slices were prepared from the hippocampus of 4–6 month-old WT-GFP and WT-JAK2ca mice. b-c, Representative paired-pulse stimulation traces for WT-GFP and WT-JAK2ca mice (100 ms interval). JAK2ca shifts the input/output relationship to the right, reducing the strength of basal glutamatergic transmission (b, Two way ANOVA and Bonferroni test) without impacting release probability, as revealed by unchanged PPR at 50 V (c, Student t test). N = 15–13. d, Representative (left) and average (right) fEPSPs before (1) and after (2) HFS protocol. LTP is impaired in WT-JAK2ca mice. e, Bar graph representing normalized fEPSP slopes 40 to 50 min post HFS. N = 8 in each group. Student t test. * p < 0.05, *** p < 0.001
Discussion
In this study, we combined astrocyte-targeted viral gene transfer, histology, biochemical analysis, cell sorting, astrocyte-specific transcriptomics, behavioral analysis and electrophysiology, in mouse models of AD and naïve WT mice. We used two transgenic mouse models of AD that recapitulate amyloid deposition, synaptic and behavioral alterations characteristic of AD, as well as progressive astrocyte reactivity including morphological and molecular changes. We found that inhibition of the JAK2-STAT3 pathway normalizes several features of astrocyte reactivity and improves three key pathological hallmarks in AD mouse models, showing that reactive astrocytes have deleterious effects in AD (Fig. 9).
The JAK2-STAT3 pathway is a master regulator of astrocyte reactivity that contributes to AD deficits. SOCS3-mediated inhibition of this cascade in AD mouse models blocked and even reversed morphological and molecular hallmarks of reactivity. Conversely, activation of the JAK2-STAT3 pathway by viral gene transfer of JAK2ca in WT mice was sufficient to induce those hallmarks. Inhibition of this cascade in AD mice reduced amyloid deposition, deficits in spatial learning and synaptic dysfunction, showing that reactive astrocytes significantly contribute to AD pathological outcomes
The JAK2-STAT3 pathway controls astrocyte reactivity
We previously reported that STAT3 is activated in reactive astrocytes in several mouse and primate models of ND [8], and the active, phosphorylated form of STAT3 is found in some hippocampal GFAP+ astrocytes in AD patients [81]. STAT3 is also activated in reactive astrocytes in acute CNS diseases such as ischemia, or spinal cord injury [12, 61]. Many molecular triggers are able to activate STAT3 (e.g. cytokines, growth factors, as well as ephrins [80] or the Notch pathway [41]). Therefore, despite the variety of pathological stimuli associated with these disorders, they appear to converge, either directly or indirectly, on STAT3, making STAT3 activation a universal feature of astrocyte reactivity.
We used viral gene transfer in the mouse brain to inhibit or activate the JAK2-STAT3 pathway in astrocytes and identified this cascade as a master regulator of astrocyte reactivity. We show that overexpression of a constitutively active form of JAK2 in WT astrocytes is sufficient to trigger robust astrocyte reactivity in the hippocampus, in the absence of pathological stimulus. By expressing the inhibitor SOCS3 in astrocytes, we also showed that the JAK2-STAT3 pathway controls astrocyte reactivity in two mouse models of AD. Interestingly, SOCS3 was not only able to prevent, but also to reverse astrocyte reactivity in the hippocampus of aged APP mice. Thus, activation of the JAK2-STAT3 pathway is necessary both for the induction and persistence of many aspects of astrocyte reactivity (Fig. 9). Contrary to conditional approaches used in acute injury models [4, 26, 61], our SOCS3-based strategy to block reactivity has the advantage of inhibiting only the JAK-dependent effects of STAT3, without impacting other non-canonical functions of STAT3 that do not require phosphorylation on Tyrosine 705 [12]. In addition, our virus-based method provides a local and time-controlled inhibition of the JAK-STAT3 pathway, avoiding peripheral or developmental side effects. SOCS3 is a specific inhibitor of the JAK-STAT3 cascade thanks to its dual recognition of JAK and the phosphorylated motif on the cytokine receptor [6, 35]. We controlled that SOCS3 overexpression did not affect other signaling cascades. We did not find evidence for ERK or STAT1 activation in APP astrocytes and no further effect of SOCS3, suggesting that STAT3 is the main transcription factor regulated by SOCS3 in our conditions.
We found that SOCS3 regulates many genes linked to inflammation and immunity that are induced in astrocytes of AD mouse models (our data and [45, 62]) and in astrocytes of AD patient brains [62, 73]. In particular, several genes encoding cytokines and complement factors, which are associated with synaptic alterations and molecular defects characteristic of AD [25, 45, 78], were down-regulated by SOCS3. Interestingly, the induction of some SOCS3-regulated genes was shown to be dependent on STAT3, in a model of spinal cord injury in mice with an astrocyte-specific knock out of STAT3 [4]. Our study is of high therapeutic relevance for AD as it identifies the cascade controlling the transcription of neuroinflammatory genes by astrocytes. It will be important to measure how those inflammatory mediators are impacted by SOCS3 at the protein level.
Besides the JAK2-STAT3 pathway, other signaling cascades were reportedly activated during ND [7, 33]. But only a few cascades have been specifically manipulated in astrocytes to test their requirement for reactivity during AD. Constitutive activation of calcineurin in APP mice decreases astrocyte reactivity [19]. Surprisingly, the opposite manipulation of the calcineurin/Nuclear Factor of Activated T-cells (NFAT) pathway by expression of a blocking peptide in astrocytes also attenuates reactivity in the same mouse model [22], questioning the regulatory action of this pathway. Activation of the Nuclear Factor of κ light polypeptide gene enhancer in B-cells (NF-κB) pathway in astrocytes, through a conditional knock-out of the IκBα inhibitor or by expression of a constitutively active IKK, increases glial reactivity in the cortex and hippocampus of WT and AD mice [44, 60]. But, to our knowledge, the specific inhibition of this cascade in astrocytes has not been performed in AD models. Overall, modulation of calcineurin/NFAT or NF-κB pathways appear to have inconsistent, transient or moderate effects on astrocyte reactivity in AD models. On the contrary, we studied two mouse models of AD (APP and 3xTg) and observed consistent effects of JAK2-STAT3 pathway modulation on astrocyte reactivity. Importantly, these effects were remarkably stable over time, lasting up to 9 months post-injection.
The concept of reactive astrocyte heterogeneity is emerging [3, 47], especially with the recent description of A1 and A2 subtypes of reactive astrocytes [48, 68, 76, 84]. Reactive astrocytes may thus form discrete subtypes with specific molecular and functional properties. The precise signaling pathways controlling these reactive states are still unknown. Unexpectedly, we found that SOCS3 regulates the expression of pan, A1 and A2 specific genes. In fact, the core WGCNA module of SOCS3-regulated genes contained all types of reactive markers. These results suggest that SOCS3 mediates a global inhibition of astrocyte reactivity, which operates beyond specific classes of reactive astrocytes. In this study, we did not explore the effects of SOCS3 in WT astrocytes, it will be interesting to see whether SOCS3 also regulates the transcriptome of astrocytes and some of their functions when they are not in an AD pathological environment.
Surprisingly, we found that inhibition of astrocyte reactivity in APP mice by SOCS3 did not impact the molecular and functional features of microglial cells. Microglia and astrocytes are engaged in complex bidirectional communications [25]. In particular, reactive microglial cells are reported to play a key role in triggering astrocyte reactivity in inflammatory conditions [70]. Our results, suggest that, at least in AD, reactive astrocytes do not significantly regulate microglial activation. Microglial cells may be already strongly activated by toxic amyloid proteins and neuronal dysfunction as occurring in AD.
Reactive astrocytes contribute to pathological outcomes in AD models
We found that inhibition of astrocyte reactivity by SOCS3 reduces the number of amyloid plaques in APP mice, a key pathological hallmark of AD. Intriguingly, the average size of plaques and the overall size distribution were not changed, suggesting that SOCS3 reduces amyloid seeding into plaques, but once amyloid plaques form, their growth and evolution are not impacted. Amyloid production, aggregation and clearance involve several brain cell types, including neurons that produce the bulk of Aβ peptides and microglial cells that actively degrade amyloid plaques. Astrocytes may produce low levels of Aβ as well [46] and are able to degrade amyloid plaques [37, 79, 82]. However, our FACS analysis evidenced no Aβ phagocytosis in astrocytes, and RNAseq data showed no increase in the expression of phagocytic receptors in astrocytes, suggesting that SOCS3-astrocytes do not contribute to Aβ phagocytosis. In addition, we ruled out microglial involvement in enhanced Aβ clearance in the SOCS3 group. Last, stable levels of soluble Aβ40 and Aβ42, as well as proteins involved in APP metabolism, suggest that Aβ production is not altered by JAK2-STAT3 pathway modulation in astrocytes. The molecular and cellular mechanisms responsible for the reduced number of amyloid plaques with SOCS3 are still unknown. Astrocytes were recently shown to contribute to Aβ elimination through the glymphatic system, which allows perivascular drainage of interstitial fluid to remove soluble brain waste [29]. SOCS3 could improve this clearance mechanism, thereby diverting soluble Aβ from aggregating into plaques. It is however quite difficult to test this hypothesis, as available methods to probe the glymphatic system lack spatial resolution to quantify SOCS3 local effects in the hippocampus.
We also report that SOCS3-mediated inhibition of astrocyte reactivity restores spatial learning on the Morris water maze, without correcting deficits in memory retrieval at 72 h. It is striking that expression of SOCS3 in only 25% of hippocampal astrocytes is able to restore spatial learning. Spatial learning is a hippocampal-dependent task [56], and it is now well documented that astrocytes are able to regulate hippocampal circuits and impact behavior [1, 66, 69]. However, the local action of AAV on hippocampal astrocytes may explain why long term memory was not corrected by AAV-SOCS3 in APP mice, because storage and retrieval of spatial memories mostly involve neocortical regions, which are not targeted by AAVs [52]. The finding that hippocampal astrocyte reactivity contributes to learning deficits in APP mice and is amenable to restoration by SOCS3 opens new therapeutic applications for AD.
Further demonstrating SOCS3 therapeutic potential, we found that its expression in astrocytes fully restored early synaptic and LTP alterations in 3xTg mice. Partial reduction in astrocyte reactivity by NFAT inhibition also improves synaptic transmission in aged APP mice [22]. Here, we uncover an implication of reactive astrocytes in the initial stages of synaptic dysfunction, before overt amyloid and Tau pathology, stressing the importance of astrocytes as therapeutic targets for AD. In accordance, we found that induction of astrocyte reactivity by JAK2ca was sufficient to induce synaptic deficits in WT mice, similar to those observed in 3xTg mice. Astrocytes regulate synaptic transmission by promoting ion homeostasis, recycling neurotransmitters or releasing active molecules such as gliotransmitters [5, 64, 65]. Some of these mechanisms are altered in AD mouse models [7, 15, 31] and could be restored by SOCS3. In addition, mRNA levels of several synaptotoxic complement factors [45, 78] were reduced by SOCS3. It is thus possible that SOCS3 normalizes several aspects of neuron-astrocyte interactions at the synapse, and further experiments will be needed to identify the exact mechanisms at play. Our model of JAK2ca-induced astrocyte reactivity will be helpful to isolate astrocyte-specific mechanisms of synaptic impairment.
The fact that JAK2-STAT3-mediated astrocyte reactivity contributes to three AD pathological outcomes contrasts with the beneficial effects of astrocytic STAT3 in spinal cord injury [4] or lack of effects after chemical lesions [58]. It is possible that some, but not all, astrocyte functions are regulated by this pathway. Depending on the disease context, STAT3-mediated astrocyte reactivity may thus have variable effects.
Conclusion
We show that the JAK2-STAT3 pathway is a core signaling cascade for the induction and maintenance of astrocyte reactivity. This pathway controls key morphological features and coordinates gene expression in reactive astrocytes. The JAK2-STAT3 pathway is a potent molecular target to establish the overall role of reactive astrocytes in CNS diseases. Our results show that in AD, reactive astrocytes are mostly deleterious, contributing to amyloid deposition, spatial learning deficits and synaptic dysfunction. Blocking astrocyte reactivity via the JAK2-STAT3 pathway offers new therapeutic opportunities for AD.
References
Adamsky A, Kol A, Kreisel T, Doron A, Ozeri-Engelhard N, Melcer T et al (2018) Astrocytic activation generates De novo neuronal potentiation and memory enhancement. Cell 174:59–71 e14. https://doi.org/10.1016/j.cell.2018.05.002
Allaman I, Gavillet M, Belanger M, Laroche T, Viertl D, Lashuel HA et al (2010) Amyloid-beta aggregates cause alterations of astrocytic metabolic phenotype: impact on neuronal viability. J Neurosci 30:3326–3338. https://doi.org/10.1523/JNEUROSCI.5098-09.2010
Anderson MA, Ao Y, Sofroniew MV (2014) Heterogeneity of reactive astrocytes. Neurosci Lett 565:23–29. https://doi.org/10.1016/j.neulet.2013.12.030
Anderson MA, Burda JE, Ren Y, Ao Y, O'Shea TM, Kawaguchi R et al (2016) Astrocyte scar formation aids central nervous system axon regeneration. Nature 532:195–200. https://doi.org/10.1038/nature17623
Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A (2014) Gliotransmitters travel in time and space. Neuron 81:728–739. https://doi.org/10.1016/j.neuron.2014.02.007
Babon JJ, Kershaw NJ, Murphy JM, Varghese LN, Laktyushin A, Young SN et al (2012) Suppression of cytokine signaling by SOCS3: characterization of the mode of inhibition and the basis of its specificity. Immunity 36:239–250. https://doi.org/10.1016/j.immuni.2011.12.015
Ben Haim L, Carrillo-de Sauvage MA, Ceyzeriat K, Escartin C (2015) Elusive roles for reactive astrocytes in neurodegenerative diseases. Front Cell Neurosci 9:278. https://doi.org/10.3389/fncel.2015.00278
Ben Haim L, Ceyzeriat K, Carrillo-de Sauvage MA, Aubry F, Auregan G, Guillermier M et al (2015) The JAK/STAT3 pathway is a common inducer of astrocyte reactivity in Alzheimer's and Huntington's diseases. J Neurosci 35:2817–2829. https://doi.org/10.1523/JNEUROSCI.3516-14.2015
Burda JE, Sofroniew MV (2014) Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81:229–248. https://doi.org/10.1016/j.neuron.2013.12.034
Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS et al (2008) A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28:264–278. https://doi.org/10.1523/JNEUROSCI.4178-07.2008
Cantarella G, Di Benedetto G, Puzzo D, Privitera L, Loreto C, Saccone S et al (2015) Neutralization of TNFSF10 ameliorates functional outcome in a murine model of Alzheimer's disease. Brain 138:203–216. https://doi.org/10.1093/brain/awu318
Ceyzeriat K, Abjean L, Carrillo-de Sauvage MA, Ben Haim L, Escartin C (2016) The complex STATes of astrocyte reactivity: how are they controlled by the JAK-STAT3 pathway? Neuroscience 330:205–218. https://doi.org/10.1016/j.neuroscience.2016.05.043
Chun H, Lee CJ (2018) Reactive astrocytes in Alzheimer's disease: a double-edged sword. Neurosci Res 126:44–52. https://doi.org/10.1016/j.neures.2017.11.012
Clarke LE, Liddelow SA, Chakraborty C, Munch AE, Heiman M, Barres BA (2018) Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci U S A 115:E1896–E1905. https://doi.org/10.1073/pnas.1800165115
Delekate A, Fuchtemeier M, Schumacher T, Ulbrich C, Foddis M, Petzold GC (2014) Metabotropic P2Y1 receptor signalling mediates astrocytic hyperactivity in vivo in an Alzheimer's disease mouse model. Nat Commun 5:5422. https://doi.org/10.1038/ncomms6422
Ding Y, Qiao A, Wang Z, Goodwin JS, Lee ES, Block ML et al (2008) Retinoic acid attenuates beta-amyloid deposition and rescues memory deficits in an Alzheimer's disease transgenic mouse model. J Neurosci 28:11622–11634. https://doi.org/10.1523/JNEUROSCI.3153-08.2008
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S et al (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29:15–21. https://doi.org/10.1093/bioinformatics/bts635
Escartin C, Brouillet E, Gubellini P, Trioulier Y, Jacquard C, Smadja C et al (2006) Ciliary neurotrophic factor activates astrocytes, redistributes their glutamate transporters GLAST and GLT-1 to raft microdomains, and improves glutamate handling in vivo. J Neurosci 26:5978–5989
Fernandez AM, Jimenez S, Mecha M, Davila D, Guaza C, Vitorica J et al (2012) Regulation of the phosphatase calcineurin by insulin-like growth factor I unveils a key role of astrocytes in Alzheimer's pathology. Mol Psychiatry 17:705–718. https://doi.org/10.1038/mp.2011.128
Ferreira TA, Blackman AV, Oyrer J, Jayabal S, Chung AJ, Watt AJ et al (2014) Neuronal morphometry directly from bitmap images. Nat Methods 11:982–984. https://doi.org/10.1038/nmeth.3125
Fol R, Braudeau J, Ludewig S, Abel T, Weyer SW, Roederer JP et al (2016) Viral gene transfer of APPsalpha rescues synaptic failure in an Alzheimer's disease mouse model. Acta Neuropathol 131:247–266. https://doi.org/10.1007/s00401-015-1498-9
Furman JL, Sama DM, Gant JC, Beckett TL, Murphy MP, Bachstetter AD et al (2012) Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer's disease. J Neurosci 32:16129–16140. https://doi.org/10.1523/JNEUROSCI.2323-12.2012
Haan S, Wuller S, Kaczor J, Rolvering C, Nocker T, Behrmann I et al (2009) SOCS-mediated downregulation of mutant Jak2 (V617F, T875N and K539L) counteracts cytokine-independent signaling. Oncogene 28:3069–3080
Hardy JA, Higgins GA (1992) Alzheimer's disease: the amyloid cascade hypothesis. Science 256:184–185
Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL et al (2015) Neuroinflammation in Alzheimer's disease. Lancet Neurol 14:388–405. https://doi.org/10.1016/S1474-4422(15)70016-5
Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK et al (2008) STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci 28:7231–7243
Hol EM, Pekny M (2015) Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol 32:121–130. https://doi.org/10.1016/j.ceb.2015.02.004
Huang da W, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57. https://doi.org/10.1038/nprot.2008.211
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA et al (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 4:147ra111. https://doi.org/10.1126/scitranslmed.3003748
Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA et al (2004) Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet 13:159–170. https://doi.org/10.1093/hmg/ddh019
Jo S, Yarishkin O, Hwang YJ, Chun YE, Park M, Woo DH et al (2014) GABA from reactive astrocytes impairs memory in mouse models of Alzheimer's disease. Nat Med. https://doi.org/10.1038/nm.3639
Kamphuis W, Kooijman L, Orre M, Stassen O, Pekny M, Hol EM (2015) GFAP and vimentin deficiency alters gene expression in astrocytes and microglia in wild-type mice and changes the transcriptional response of reactive glia in mouse model for Alzheimer's disease. Glia. https://doi.org/10.1002/glia.22800
Kang W, Hebert JM (2011) Signaling pathways in reactive astrocytes, a genetic perspective. Mol Neurobiol 43:147–154. https://doi.org/10.1007/s12035-011-8163-7
Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK et al (2017) A unique microglia type associated with restricting development of Alzheimer's disease. Cell 169:1276–1290 e1217. https://doi.org/10.1016/j.cell.2017.05.018
Kershaw NJ, Murphy JM, Liau NP, Varghese LN, Laktyushin A, Whitlock EL et al (2013) SOCS3 binds specific receptor-JAK complexes to control cytokine signaling by direct kinase inhibition. Nat Struct Mol Biol 20:469–476. https://doi.org/10.1038/nsmb.2519
Kim D, Kim SH, Cho SH, Shin K, Kim S (2011) SOCS3 suppresses the expression of IL-4 cytokine by inhibiting the phosphorylation of c-Jun through the ERK signaling pathway in rat mast cell line RBL-2H3. Mol Immunol 48:776–781. https://doi.org/10.1016/j.molimm.2010.11.005
Koistinaho M, Lin S, Wu X, Esterman M, Koger D, Hanson J et al (2004) Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat Med 10:719–726. https://doi.org/10.1038/nm1058
Kraft AW, Hu X, Yoon H, Yan P, **ao Q, Wang Y et al (2013) Attenuating astrocyte activation accelerates plaque pathogenesis in APP/PS1 mice. FASEB J 27:187–198. https://doi.org/10.1096/fj.12-208660
Krauthausen M, Kummer MP, Zimmermann J, Reyes-Irisarri E, Terwel D, Bulic B et al (2015) CXCR3 promotes plaque formation and behavioral deficits in an Alzheimer's disease model. J Clin Invest 125:365–378. https://doi.org/10.1172/JCI66771
Langfelder P, Horvath S (2008) WGCNA: an R package for weighted correlation network analysis. BMC Bioinform 9:559. https://doi.org/10.1186/1471-2105-9-559
LeComte MD, Shimada IS, Sherwin C, Spees JL (2015) Notch1-STAT3-ETBR signaling axis controls reactive astrocyte proliferation after brain injury. Proc Natl Acad Sci U S A 112:8726–8731. https://doi.org/10.1073/pnas.1501029112
Lee CY, Landreth GE (2010) The role of microglia in amyloid clearance from the AD brain. J Neural Transm 117:949–960. https://doi.org/10.1007/s00702-010-0433-4
Lee Y, Messing A, Su M, Brenner M (2008) GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia 56:481–493. https://doi.org/10.1002/glia.20622
Lian H, Litvinchuk A, Chiang AC, Aithmitti N, Jankowsky JL, Zheng H (2016) Astrocyte-microglia cross talk through complement activation modulates amyloid pathology in mouse models of Alzheimer's disease. J Neurosci 36:577–589. https://doi.org/10.1523/JNEUROSCI.2117-15.2016
Lian H, Yang L, Cole A, Sun L, Chiang AC, Fowler SW et al (2015) NFkappaB-activated Astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer's disease. Neuron 85:101–115. https://doi.org/10.1016/j.neuron.2014.11.018
Liao MC, Muratore CR, Gierahn TM, Sullivan SE, Srikanth P, De Jager PL et al (2016) Single-cell detection of secreted Abeta and sAPPalpha from human IPSC-derived neurons and astrocytes. J Neurosci 36:1730–1746. https://doi.org/10.1523/JNEUROSCI.2735-15.2016
Liddelow SA, Barres BA (2017) Reactive astrocytes: production, function, and therapeutic potential. Immunity 46:957–967. https://doi.org/10.1016/j.immuni.2017.06.006
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487. https://doi.org/10.1038/nature21029
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. https://doi.org/10.1186/s13059-014-0550-8
Marchetti C, Marie H (2011) Hippocampal synaptic plasticity in Alzheimer's disease: what have we learned so far from transgenic models? Rev Neurosci 22:373–402. https://doi.org/10.1515/RNS.2011.035
Masliah E, Alford M, DeTeresa R, Mallory M, Hansen L (1996) Deficient glutamate transport is associated with neurodegeneration in Alzheimer's disease. Ann Neurol 40:759–766. https://doi.org/10.1002/ana.410400512
Maviel T, Durkin TP, Menzaghi F, Bontempi B (2004) Sites of neocortical reorganization critical for remote spatial memory. Science 305:96–99. https://doi.org/10.1126/science.1098180
McManus MJ, Murphy MP, Franklin JL (2011) The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer's disease. J Neurosci 31:15703–15715. https://doi.org/10.1523/JNEUROSCI.0552-11.2011
Mertens C, Darnell JE Jr (2007) SnapShot: JAK-STAT signaling. Cell 131:612. https://doi.org/10.1016/j.cell.2007.10.033
Morizawa YM, Hirayama Y, Ohno N, Shibata S, Shigetomi E, Sui Y et al (2017) Reactive astrocytes function as phagocytes after brain ischemia via ABCA1-mediated pathway. Nat Commun 8:28. https://doi.org/10.1038/s41467-017-00037-1
Morris RG, Garrud P, Rawlins JN, O'Keefe J (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297:681–683
Noli L, Capalbo A, Ogilvie C, Khalaf Y, Ilic D (2015) Discordant growth of monozygotic twins starts at the blastocyst stage: a case study. Stem Cell Reports 5:946–953. https://doi.org/10.1016/j.stemcr.2015.10.006
O'Callaghan JP, Kelly KA, VanGilder RL, Sofroniew MV, Miller DB (2014) Early activation of STAT3 regulates reactive astrogliosis induced by diverse forms of neurotoxicity. PLoS One 9:e102003. https://doi.org/10.1371/journal.pone.0102003
Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R et al (2003) Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron 39:409–421 Doi: S0896627303004343
Oeckl P, Lattke M, Wirth T, Baumann B, Ferger B (2012) Astrocyte-specific IKK2 activation in mice is sufficient to induce neuroinflammation but does not increase susceptibility to MPTP. Neurobiol Dis 48:481–487. https://doi.org/10.1016/j.nbd.2012.06.010
Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K et al (2006) Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med 12:829–834
Orre M, Kamphuis W, Osborn LM, Jansen AH, Kooijman L, Bossers K et al (2014) Isolation of glia from Alzheimer's mice reveals inflammation and dysfunction. Neurobiol Aging 35:2746–2760. https://doi.org/10.1016/j.neurobiolaging.2014.06.004
Osborn LM, Kamphuis W, Wadman WJ, Hol EM (2016) Astrogliosis: an integral player in the pathogenesis of Alzheimer's disease. Prog Neurobiol 144:121–141. https://doi.org/10.1016/j.pneurobio.2016.01.001
Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA et al (2006) Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell 125:775–784
Panatier A, Vallee J, Haber M, Murai KK, Lacaille JC, Robitaille R (2011) Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 146:785–798 Doi: S0092-8674(11)00820-8 [pii]. https://doi.org/10.1016/j.cell.2011.07.022
Papouin T, Dunphy JM, Tolman M, Dineley KT, Haydon PG (2017) Septal cholinergic Neuromodulation tunes the astrocyte-dependent gating of hippocampal NMDA receptors to wakefulness. Neuron 94:840–854 e847. https://doi.org/10.1016/j.neuron.2017.04.021
Pekny M, Pekna M, Messing A, Steinhauser C, Lee JM, Parpura V et al (2015) Astrocytes: a central element in neurological diseases. Acta Neuropathol. https://doi.org/10.1007/s00401-015-1513-1
Priego N, Zhu L, Monteiro C, Mulders M, Wasilewski D, Bindeman W et al (2018) STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat Med. https://doi.org/10.1038/s41591-018-0044-4
Robin LM, Oliveira da Cruz JF, Langlais VC, Martin-Fernandez M, Metna-Laurent M, Busquets-Garcia A et al (2018) Astroglial CB1 receptors determine synaptic D-serine availability to enable recognition memory. Neuron 98:935–944 e935. https://doi.org/10.1016/j.neuron.2018.04.034
Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao CC, Ardura-Fabregat A et al (2018) Microglial control of astrocytes in response to microbial metabolites. Nature 557:724–728. https://doi.org/10.1038/s41586-018-0119-x
Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L et al (2016) Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med 22:586–597. https://doi.org/10.1038/nm.4106
Sancheti H, Patil I, Kanamori K, Diaz Brinton R, Zhang W, Lin AL et al (2014) Hypermetabolic state in the 7-month-old triple transgenic mouse model of Alzheimer's disease and the effect of lipoic acid: a 13C-NMR study. J Cereb Blood Flow Metab 34:1749–1760. https://doi.org/10.1038/jcbfm.2014.137
Sekar S, McDonald J, Cuyugan L, Aldrich J, Kurdoglu A, Adkins J et al (2015) Alzheimer's disease is associated with altered expression of genes involved in immune response and mitochondrial processes in astrocytes. Neurobiol Aging 36:583–591. https://doi.org/10.1016/j.neurobiolaging.2014.09.027
Selkoe DJ (2002) Alzheimer's disease is a synaptic failure. Science 298:789–791. https://doi.org/10.1126/science.1074069
Sharma K, Schmitt S, Bergner CG, Tyanova S, Kannaiyan N, Manrique-Hoyos N et al (2015) Cell type- and brain region-resolved mouse brain proteome. Nat Neurosci 18:1819–1831. https://doi.org/10.1038/nn.4160
Shi Y, Yamada K, Liddelow SA, Smith ST, Zhao L, Luo W et al (2017) ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 549:523–527. https://doi.org/10.1038/nature24016
Sompol P, Furman JL, Pleiss MM, Kraner SD, Artiushin IA, Batten SR et al (2017) Calcineurin/NFAT signaling in activated astrocytes drives network Hyperexcitability in Abeta-bearing mice. J Neurosci 37:6132–6148. https://doi.org/10.1523/JNEUROSCI.0877-17.2017
Stephan AH, Barres BA, Stevens B (2012) The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci 35:369–389. https://doi.org/10.1146/annurev-neuro-061010-113810
Thal DR, Hartig W, Schober R (1999) Diffuse plaques in the molecular layer show intracellular a beta(8-17)-immunoreactive deposits in subpial astrocytes. Clin Neuropathol 18:226–231
Tyzack GE, Hall CE, Sibley CR, Cymes T, Forostyak S, Carlino G et al (2017) A neuroprotective astrocyte state is induced by neuronal signal EphB1 but fails in ALS models. Nat Commun 8:1164. https://doi.org/10.1038/s41467-017-01283-z
Wan J, Fu AK, Ip FC, Ng HK, Hugon J, Page G et al (2010) Tyk2/STAT3 signaling mediates beta-amyloid-induced neuronal cell death: implications in Alzheimer's disease. J Neurosci 30:6873–6881. https://doi.org/10.1523/JNEUROSCI.0519-10.2010
Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F et al (2003) Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med 9:453–457
Ye B, Shen H, Zhang J, Zhu YG, Ransom BR, Chen XC et al (2015) Dual pathways mediate beta-amyloid stimulated glutathione release from astrocytes. Glia 63:2208–2219. https://doi.org/10.1002/glia.22886
Yun SP, Kam TI, Panicker N, Kim S, Oh Y, Park JS et al (2018) Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson's disease. Nat Med. https://doi.org/10.1038/s41591-018-0051-5
Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG et al (2012) Genomic analysis of reactive astrogliosis. J Neurosci 32:6391–6410. https://doi.org/10.1523/JNEUROSCI.6221-11.2012
Acknowledgments
We thank Drs. N. Robil and P. de la Grange from Genosplice Technology® for bioinformatics analysis of RNAseq data. We thank Profs. Haan, Bjørbæk and Shoelson for sharing JAK2ca and SOCS3 plasmids. We thank Drs. S. Alves and N. Cartier for sharing their IDE antibody. We thank L. Vincent, K. Bastide, P. Woodling and J.M. Hélies for their help with mouse housing and transfer to Bordeaux; C. Garin and C. Dudeffant for help with the APP/PS1dE9 colony. We are grateful to F. Aubry for help with initial AAV vector cloning and FACS experiments; D. Cheramy and Dr. E. Diguet (Servier) for sharing their expertise and equipment for MSD® kits; Dr. L. Juricek and Dr. C. Jan for help with pilot experiments and Prof. N. Déglon for technical input at the initial part of the project. We acknowledge the help of V. Lavilla and C. Dulary on transcriptomic studies; D. Bacq and the QC team, the informatic and bio-informatic teams at the CNRGH. We thank D. Gonzales, S. Laumond, J. Tessaire and the NeuroCentre Magendie mouse facility staff for mouse care and genoty**. We are grateful to Dr. L. Schirmer for his comments and careful reading of an earlier version of the manuscript.
Funding
This study was supported by CEA, CNRS and grants from the French National Research Agency (grants # 2010-JCJC-1402-1, 2011-BSV4–021-03 and ANR-16-TERC-0016-01 to C.E. and 2011-INBS-0011 NeurATRIS to P.H.), from Fondation Vaincre Alzheimer (grant # FR-15015, to C. E.), Laboratory of Excellence in Medical Genomics: GENMED (to J-F.D.) as well as CNRS and INSERM (to S.H.R.O. and A.P) and Association France Alzheimer and Fondation de France (Prix Spécial 2012 to G.B.). C.E. and A.P. received support from the Fédération pour la Recherche sur le Cerveau. K. Ceyzériat, L. Ben Haim and O. Guillemaud are recipients of a doctoral fellowship from the CEA.
Availability of data and materials
RNAseq dataset is deposited on the GEO server under reference GSE108520.
Author information
Authors and Affiliations
Contributions
KCe, LBH, performed experiments, analyzed data and wrote the manuscript. AD, DP, MM, VL, JV performed and analyzed electrophysiological recordings. OG, LA performed experiments and analyzed data. MAP performed RNAseq experiments. PG, FP performed some histological stainings. MCG contributed to primer design and vector cloning. MGu, SB, GA, MGa helped with mouse surgery and breeding. CJ produced viral vectors. ND, JB performed cell sorting. KCa supervised the Morris water maze test. ABe supervised viral vector production. GB provided 3xTg mice. MD provided APP mice. GB, SHRO, JFD, PH, EB provided additional funding. MACS performed experiments, analyzed data and supervised some experiments. RO designed and supervised transcriptomic experiments. AP designed, analyzed and supervised electrophysiological recordings and wrote the manuscript. CE designed the study, obtained funding, analyzed data and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experimental protocols were reviewed and approved by the local ethics committee (CETEA N°44) and submitted to the French Ministry of Education and Research (Approvals # APAFIS#4565–20 16031711426915 v3, APAFIS#4503–2016031409023019). They were performed in a facility authorized by local authorities (authorization #B92–032-02), in strict accordance with recommendations of the European Union (2010–63/EEC).
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Figure S1. AAV infect astrocytes selectively. To validate astrocyte tropism of the AAVs used in our study, an AAV2/9 encoding GFP was injected in the hippocampus of WT mice. GFP+ cells co-express the astrocytic marker GFAP and S100β, but not NeuN, IBA1, Olig2 and MBP, which are markers of neurons, microglial cells, cells of the oligodendrocyte lineage and myelinating oligodendrocytes, respectively. Astrocyte tropism of these vectors was confirmed in AD mice as well (See colocalization of GFP with GFAP in Figs. 1b and S2). (TIF 14477 kb)
Additional file 2:
Table S1. Sequences of primers used for qPCR. (DOCX 17 kb)
Additional file 3:
FigureS2. Unlike STAT3, STAT1 and Erk are not activated in APP astrocytes. Confocal images of stained hippocampal sections from 12-month-old WT-GFP, APP-GFP and APP-SOCS3 mice. a, GFP+ astrocytes (green) stained for GFAP (magenta) and STAT3 (cyan). APP astrocytes are reactive (hypertrophic and GFAP overexpression). They display STAT3 nuclear accumulation. SOCS3 reduces GFAP and STAT3 expression in APP mice, even around amyloid plaques (star). b, Quantification of STAT3 immunoreactivity in astrocyte soma. N = 5–7/group. One way ANOVA and Tukey’s post hoc test. *** p < 0.001. c-d, Sections stained in magenta for STAT1 (c) or P-ERK (d), DAPI (blue) and GFP (green). STAT1 and P-ERK are not induced in APP reactive astrocytes while CNTF induces significant STAT1 nuclear accumulation (c) and LPS triggers ERK phosphorylation (d). DAPI stains nuclei as well as amyloid plaques. Representative images from N = 4–6/group. (TIF 15218 kb)
Additional file 4:
Figure S3. Validation of astrocyte sorting. Normalized expression of cell type specific genes. GFP+ astrocytes are enriched in astrocyte markers while GFP− cells, which comprise uninfected astrocytes, neurons, microglial cells and oligodendrocyte precursor cells (OPC) are enriched in other cell type markers. Oligodendocyte markers are undetectable due to the myelin removal step. N = 3–7/group. Wald test. * p < 0.05, ** p < 0.01, *** p < 0.001, # p < 10− 20, ### p < 10− 40. (TIF 12358 kb)
Additional file 5:
Table S2. WGCNA: Top 20 most connected genes regulated by SOCS3 in APP astrocytes. Three of the top 20 hub genes, highlighted in bold, are pan or A1 reactive astrocyte genes. (DOCX 17 kb)
Additional file 6:
Figure S4. SOCS3 regulates gene networks linked to reactivity in APP astrocytes. The top 100 most connected genes of the WGCNA module were analyzed for protein-protein interaction networks with STRING. Groups of proteins related to complement system and inflammation, cytoskeleton and cell adhesion were found co-regulated by SOCS3 (circles). Protein-protein interaction p value < 10− 6. (TIF 54618 kb)
Additional file 7:
Figure S5. Modulation of the JAK2-STAT3 pathway does not change expression of proteins involved in Aβ production and clearance in APP mice. Representative western blottings on protein homogenates prepared from WT-GFP, APP-GFP, APP-SOCS3 and APP-JAK2ca mice. The expression of human (hAPP) (a), BACE1 (b), IDE (c) and ApoE (d) are stable across the groups (protein levels are normalized by actin or tubulin α). N = 3–8/group. a, Student t test, b-d, ANOVA or Kruskall-Wallis tests. (TIF 10130 kb)
Additional file 8:
Table S3. Expression of phagocytic receptors and genes in astrocytes RNAseq analysis of astrocytes shows that most genes involved in Aβ phagocytosis are expressed at similar levels in all groups. Only mRNA levels for three Fc receptors are down-regulated by SOCS3. Data are presented as normalized mRNA levels +/-SEM. ## p < 0.01 versus WT-GFP, ** p < 0.01, *** p < 0.001 versus APP-GFP. Wald test. (DOCX 18 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ceyzériat, K., Ben Haim, L., Denizot, A. et al. Modulation of astrocyte reactivity improves functional deficits in mouse models of Alzheimer’s disease. acta neuropathol commun 6, 104 (2018). https://doi.org/10.1186/s40478-018-0606-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40478-018-0606-1