Abstract
Furin is an important mammalian proprotein convertase that catalyzes the proteolytic maturation of a variety of prohormones and proproteins in the secretory pathway. In the brain, the substrates of furin include the proproteins of growth factors, receptors and enzymes. Emerging evidence, such as reduced FURIN mRNA expression in the brains of Alzheimer’s disease patients or schizophrenia patients, has implicated a crucial role of furin in the pathophysiology of neurodegenerative and neuropsychiatric diseases. Currently, compared to cancer and infectious diseases, the aberrant expression of furin and its pharmaceutical potentials in neurological diseases remain poorly understood. In this article, we provide an overview on the physiological roles of furin and its substrates in the brain, summarize the deregulation of furin expression and its effects in neurodegenerative and neuropsychiatric disorders, and discuss the implications and current approaches that target furin for therapeutic interventions. This review may expedite future studies to clarify the molecular mechanisms of furin deregulation and involvement in the pathogenesis of neurodegenerative and neuropsychiatric diseases, and to develop new diagnosis and treatment strategies for these diseases.
Similar content being viewed by others
Introduction
Furin is the first proprotein convertase (PC) found in mammals in 1990 [1]. It catalyzes the proteolytic maturation of large numbers of prohormones and proproteins in the secretory pathway compartments [1,2,3]. The substrates of furin include hormones, cytokines, growth factors and enzymes, which play important roles in cell proliferation, anti-apoptosis, immunity and inflammation [1]. Furin also participates in the proteolytic processing of proteins in viruses and bacteria [4], such as the maturation of SARS-CoV-2 spike protein [5,6,7]. Thus, aberrant activity of furin has been found to be associated with a strikingly diverse range of pathological events, including cancer, cardiovascular disorders, infectious diseases and neurological diseases [4, 8,9,10]. Among these disorders, the role of furin in neurological diseases is the most poorly understood.
In the brain, the proprotein substrates cleaved by furin in vivo include precursors of growth factors such as brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) [11, 12], α- and β-secretases [13, 14], multiple matrix metalloproteases (MMPs) [15, 16], and other enzymes and receptors [1, 17, 18]. Since these substrates play vital roles in neuronal survival, axon growth, dendritic development, synaptogenesis, neurodegeneration and inflammation [19,20,21,22], a stable activity of furin is crucial for maintaining the homeostasis of the central nervous system.
A growing body of evidence has suggested that alterations of furin expression and abnormal cleavage of its substrates contribute to the pathophysiological mechanisms of neurodegenerative and neuropsychiatric diseases. Reduced expression of FURIN mRNA has been found in the brains of Alzheimer’s disease (AD) patients [13], and decreased protein levels of furin are found in the cortex of AD mice [23]. The FURIN mRNA expression is decreased in the prefrontal cortex of schizophrenia (SCZ) patients [24], whereas increased protein levels of furin are found in the temporal cortex of epilepsy patients [25]. Moreover, studies have also shown that increasing furin expression in the mouse brain enhances BDNF maturation and promotes dendritic spine density and memory in transgenic mice [26], and that inhibiting furin expression reduces the spontaneous rhythmic electrical activity of cerebral neurons and suppresses epileptic seizure activity in epileptic mice [25]. These findings indicate the involvement of furin dysregulation in these neurological disorders, leading to increased interest in furin as a potential biomarker for diagnosis of or as a therapeutic target for treatment of neurological disorders.
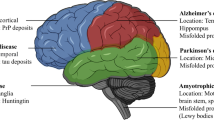
In this review, we present an overview on the physiological roles of furin in the brain and deregulations of furin expression and its substrates in neurodegenerative and neuropsychiatric disorders, such as AD, Parkinson’s disease (PD), epilepsy, cerebral ischemia, SCZ and depression. We further discuss the implications of these findings and current approaches that target furin for therapeutic interventions.
Overview of furin
Gene structure and transcription of FURIN
Furin was identified in 1990 as the first mammalian PC that catalyzes the proteolytic maturation of prohormones and proproteins of neurotrophic factors, receptors and enzymes, serum proteins and pathogen molecules [1,2,3]. The human FURIN gene is located at chromosome 15q26.1, an open reading frame upstream of the fes/fps proto-oncogene [27]. It has attracted more attention after being discovered as the first mammalian homologue of yeast Kex2 [4, 28, 29]. As shown in Fig. 1a, the human FURIN gene consists of 16 exons and encodes eight different transcript variants driven by three known promoters, P1, P1A and P1B [30, 31]. The respective transcripts differ only in the first untranslated exon and therefore generate identical furin precursor proteins [30, 32]. While the P1A and P1B promoters resemble those of constitutively expressed housekee** genes, the P1 promoter is predicted to bind to many different transcription factors, including hypoxia-inducible factor-1 (HIF-1), C/EBPβ, and CREB (cAMP-responsive element binding protein) [33,34,35,36].
Human FURIN gene and furin protein structures. a The human FURIN gene consists of 16 exons and encodes eight different transcript variants driven by three known promoters, P1, P1A and P1B. Exons are shown as green boxes and introns are shown as lines. The red boxes indicate the three promoter regions. The blue arrows indicate the positions where different transcripts start. The red arrow indicates the translational start, and the start codon (ATG) and stop codon (TGA) are marked with dotted lines. b Furin protein contains an N-terminal signal peptide, a prodomain, a subtilisin-like catalytic domain, a middle P-domain, a cysteine-rich region, a transmembrane helix domain and a C-terminal cytoplasmic domain
Several intracellular and extracellular factors have been reported to regulate FURIN expression at the transcriptional level. Hypoxia remarkably increases the expression of FURIN mRNA via stabilizing HIF-1 and enhancing its binding to hypoxia-responsive element site at the P1 promoter [37]. Iron deficiency also upregulates FURIN transcription through stabilization of HIF-1α [35], whereas iron overload inhibits furin expression in a non-HIF-1α-dependent manner [35]. Transforming growth factor beta1 (TGFβ1) can induce transactivation of the FURIN P1 promoter through binding to Sma- and Mad-related protein 2 (SMAD2) and SMAD4 in complex with other DNA-binding partners, creating a constitutive activation/regulation positive feedback loop between TGFβ1 and furin [38]. Furthermore, extracellular regulated protein kinase 1 has been found to mediate the TGFβ–furin feedback loop in glioma-initiating cells [39]. In addition, bone morphogenetic protein 2 increases the transcription and translation of furin in human granulosa lutein cells by the activin receptor-like kinase (ALK)2/ALK3-SMAD4 signaling pathway [40].
Protein structure and expression of furin
Furin is a type I transmembrane protein and belongs to the subtilisin-like convertase family [1]. It is a calcium-dependent endoserine protease [8]. Furin protein is composed of a signal peptide, a prodomain, a subtilisin-like catalytic domain, a middle P-domain, a cysteine-rich region, a transmembrane helix domain and a cytoplasmic domain (Fig. 1b) [41]. The large extracellular region of furin has an overall homology with the same region of other members of the PC family [1]. The signal peptide directs translocation of the ~ 104-kDa pro-enzyme into the endoplasmic reticulum (ER), where the first cleavage in the inhibitory prodomain takes place via autocatalytic cleavage by the catalytic domain [42,43,44]. The second cut in the prodomain is made during trafficking of the propeptide-furin complex within the mildly acidic trans-Golgi network/endosomal system, which yields the active ~ 81-kDa mature enzyme. Furin circulates between the trans-Golgi network, cell surface and endosomes, in a tightly regulated manner, to catalyze various proproteins in different cellular components [43, 45, 46].
Furin is ubiquitously expressed in vertebrates and many invertebrates [9, 47, 48]. However, its mRNA and protein levels vary depending on the tissue and cell type [49,50,51,52,53]. FURIN has been found at high mRNA levels in the salivary gland, placenta, liver and bone marrow, and high protein levels in the brain, salivary gland, pancreas, kidney and placenta [49,50,51,52,53]. However, almost no expression is detected in skin, muscle and adipose tissues [49, 50], although substrates of furin have been identified in human adipose tissues [54]. In normal single cells, high expression of FURIN mRNA is identified in hepatocytes, exocrine glandular cells, pancreatic endocrine cells and syncytiotrophoblasts [50, 53]. This tissue- and cell-specific expression pattern of furin infers the different functions of furin in different tissues and organ systems.
Function of furin
Furin cleaves proproteins at the consensus site of Arg–X–Lys/Arg–Arg or Arg–X–X–Arg (X refers to any amino acid) [55, 56], and the cut is positioned after the carboxyl-terminal Arg residue [56]. The substrates cleaved by furin include a variety of precursor proteins within the secretory pathway, including hormones, growth factors and their receptors, neuropeptides, enzymes, adhesion molecules, metalloproteinases, bacterial toxins and viral glycoproteins [8, 29, 33]. As these molecules participate in many important cellular events, mouse embryos lacking Furin will die between days 10.5 and 11.5, with notable defects in ventral closure and axial rotation [57]. Deregulations of furin expression are found in diverse pathological conditions, including cancer, diabetes, cardiovascular disorders, inflammation and neurological diseases [10, 58,123], and is upregulated in many pathological conditions, inducing neuroinflammation and apoptosis [124]. MMP-9 is specifically shown to regulate synaptic plasticity in the hippocampus by gain- and loss-of-function studies in vitro [125, 126]. Altered concentrations of MMP-3 and MMP-9 have been found in AD patients, indicating their involvement in AD pathophysiology [127]. MMP-1, MMP-2, MMP-9 and MMP-14 can cleave recombinant α-synuclein [128, 129]. Elevated levels of MMP-2 and MMP-3 have been identified in dopaminergic (DA) neurons in the substantia nigra in PD patients and animal models [129,130,131].
ADAM10
ADAMs are another major family of zinc-dependent metalloproteases involved in limited proteolysis and shedding [132]. In the brain, ADAM10 is mainly expressed in neurons [133], and is involved in the proteolytic processing of a variety of cell surface receptors and signaling molecules [134]. ADAM10 is synthesized in the ER as an inactive zymogen with a structure comprising a prodomain, a zinc-binding metalloprotease domain, a disintegrin domain, a cysteine-rich domain, a transmembrane domain and a C-terminal domain [133]. Furin cleaves the ~ 90 kDa pro-ADAM10, yielding a full-length active ADAM10 (∼65 kDa) [135], and after C-terminal shedding, a soluble ∼55-kDa ADAM10 is released [136]. ADAM10 has α-secretase activity [137]. It cleaves amyloid-β precursor protein (APP) to generate the soluble αAPP fragment (sAPPα) rather than the neurotoxic amyloid-β (Aβ), playing a protective role in AD [138].
BACE1
BACE1 is the major β-secretase that cleaves APP to generate Aβ [139]. BACE1 is a transmembrane aspartic protease, structurally similar to the pepsin family [140], containing two active catalytic site motifs in the luminal domain [141]. Like other aspartic proteases, BACE1 is synthesized as a precursor protein containing a N-terminal propeptide domain that is removed during maturation of the enzyme [142]. Furin or a furin-like PC is responsible for cleaving the BACE1 proprotein to yield the mature enzyme with the highest β-secretase activity [143]. Like APP, BACE1 is highly expressed in the brain [144]. Significant increases of BACE1 enzymatic activity and protein concentration have been detected in brain tissues, cerebrospinal fluid (CSF) and serum of AD patients and subjects with mild cognitive impairment [145,146,147]. BACE1 inhibitors have demonstrated therapeutic effects in preventing the initial cleaving events of APP in AD animal models [148,149,150,151,152,153].
Notch receptor
The Notch gene family encodes transmembrane receptors of ~ 300 kDa that are involved in cell-fate determination in vertebrates and invertebrates [154, 155]. The proteolytic processing of Notch receptor precursor is an essential step in the formation of biologically active Notch receptors. The constitutive processing of murine Notch1 requires a furin-like convertase, and mutations in the furin-cleavage site completely abolishes the proteolysis of the Notch1 receptor [155]. In the develo** brain, activation of Notch receptors upon ligand binding is involved in the preservation of neural progenitors and inhibition of neurogenesis [156, 157]. In the adult brain, Notch signaling influences neuronal apoptosis, microglial activation and synaptic plasticity [158,159,160,161]. Deregulations of Notch signaling are involved in AD, depression, epilepsy, and stroke [159,160,161,162,163].
LRP1
LRP1 is a multifunctional receptor that belongs to the low-density lipoprotein receptor family [164]. It is synthesized as a ~ 600-kDa precursor, which is cleaved by furin in the trans-Golgi network and transported to the cell surface as a mature form consisting of α-chain and β-chain [8]. The mature LRP1 is further processed by other enzymes, such as MMPs and γ-secretase, to release the intracellular domain (ICD) [8]. LRP1 is highly expressed in neurons and glia of the brain, and functions to regulate proteinase activity, cytokine activity and cholesterol metabolism [165, 166]. The ligands for LRP1 include Aβ, ApoE and activated α2-macroglobulin [167]. In addition to controlling ligand metabolism, LRP1 can also regulate signaling pathways by coupling with other cell surface receptors or proteins, such as the N-methyl-D-aspartate (NMDA) receptors [168, 169]. The ICD of LRP1 can be transported into the nucleus, where it contributes to transcriptional regulation of target genes, including interferon-γ [170]. Accumulating evidence from preclinical and animal studies indicates that LRP1 is involved in AD pathogenesis not only by regulating the metabolisms of Aβ and ApoE, but also by influencing synaptic plasticity and inflammation through Aβ-independent pathways [171]. LRP1 is detected at an abundant level in post-synaptic sites of neurons, and it interacts with several synaptic proteins, including postsynaptic density protein 95, NMDA receptor and GluA1 [169, 171,172,173]. Deletion of LRP1 in neurons has been shown to affect lipid metabolism, leptin signaling, glucose metabolism, insulin signaling and anti-apoptotic signaling, resulting in neuroinflammation, motor dysfunction, and cognitive decline in mice [171, 172, 174, 175]. In addition, LRP1 is also found to modulate stem cell proliferation and survival, astroglial differentiation [176, 177], and oligodendrocyte progenitor cell differentiation [178].
GPR37
GPR37 is an orphan G-protein-coupled receptor that is widespread in several brain regions, including cerebral cortex, hippocampus, hypothalamus, midbrain and cerebellum [51]. It has a long extracellular N-terminal ectodomain which is recently demonstrated to be processed by both ADAM10 and furin [179]. The unfolded form of GPR37 is a substrate of parkin, and its intracellular retention leads to ER stress and DA neuronal death, linking to PD [180,181,182]. GPR37 is also involved in the DA signaling pathway by interacting with the dopamine transporter in mouse striatal presynaptic membranes, thereby modulating dopamine uptake [183]. In addition, GPR37 interacts with adenosine A2A receptors in the hippocampus, localized at the extrasynaptic plasma membrane of dendritic spines, dendritic shafts and axon terminals, regulating adenosinergic signaling [184]. GPR37 is also found in astrocytes and oligodendrocytes, and is demonstrated as a negative regulator of oligodendrocyte differentiation and myelination [185, 186]. Overexpression of GPR37 leads to profound neurodegeneration in animal models, selectively for DA neurons [187], while GPR37-knockout mice also show decreased dopamine levels in the striatum and specific motor deficits [188, 189]. GPR37 knockout also triggers non-motor behavioral phenotypes, such as anxiety and depression-like behaviors, in an age- and gender-dependent manner [190, 191].
Sortilin
Sortilin is a type I transmembrane protein that functions as an endocytosis receptor and plays a role in protein sorting and cell signaling [192]. Sortilin is synthesized as an inactive precursor protein, which is cleaved by furin to remove the N-terminal propeptide [193]. The resulting mature protein can be further processed by other proteases to shed its extracellular domain from the cell surface [193]. Sortilin is generally trafficked via the trans-Golgi network, endosomes and plasma membrane, binding to different proteins and directing them to the secretory pathway or for degradation [193]. Sortilin has been reported to function as a neuronal receptor for APP and its cleavage products sAPPα and Aβ [194, 195]. The ICD of sortilin interacts with APP and regulates its lysosomal and lipid raft trafficking [194]. Sortilin also binds to oligomerized Aβ, inducing endocytosis of Aβ and triggering apoptosis [195]. In addition, sortilin is found to be an essential component for transmitting pro-neurotrophin-dependent death signals from p75NTR, thereby playing roles in neuronal apoptosis, aging and brain injury [93, 196, 197]. On the other hand, sortilin has also been found to associate with TrkB receptors, which promotes cell survival [198]. Therefore, sortilin acts as a molecular switch from apoptotic response by interacting with p75NTR to neurotrophic effects via binding to TrkB receptors in neurons. Aberrant activity of sortilin has been found to be associated with the pathogenesis of AD and depression [193, 199, 200].
BRI2
BRI2 is a type II transmembrane protein of 266 amino acids, containing an extracellular region, a transmembrane region and a cytoplasmic region [201, 202]. During maturation, the ~ 4-kDa C-terminal propeptide of BRI2 is cleaved by furin at the trans-Golgi compartment, generating the membrane-bound form of mature BRI2 (mBRI2) [203, 204]. The mBRI2 contains an evolutionarily conserved BRICHOS domain that is found to act as a chaperone, facilitating proper folding of BRI2 and preventing Aβ formation [205, 206]. In the human brain, BRI2 is intensively expressed in cortical and hippocampal pyramidal neurons [207]. The BRICHOS domain of BRI2 interacts with APP and inhibits its processing, delaying fibrillation of Aβ [206,207,208,209]. Mutations in BRI2 and aberrant BRI2 expression have been reported to be associated with familial British dementia and involved in AD pathogenesis [210,211,212].
Ac45
Ac45, an accessory subunit of the vacuolar-type ATPase (V-ATPase) proton pump, is a type I transmembrane protein that is encoded by ATP6AP1 in humans [213,214,290].
Potential role of furin in the pathology of epilepsy
The above findings suggest a crucial role of furin in the pathology of epilepsy. The upregulation of furin in epilepsy patients or animal models may promote the cleavage of proBDNF, proNGF, Notch receptor and MMPs. As a result, the inhibitory and excitatory synaptic transmissions are affected, leading to abnormal neuronal discharge, which contributes in part to the symptoms of epilepsy (Fig. 3b). However, the underlying mechanisms for furin upregulation and furin-mediated activities in epileptogenesis need to be determined.
Furin in cerebral ischemia
Overview of cerebral ischemia
Cerebral ischemia is a neurodegenerative disease caused by reduced blood supply to the brain tissue [291], and is currently a major cause of death and disability globally [292]. Cerebral ischemia causes reduced delivery of oxygen and glucose to the brain, and as a result, a loss of consciousness can occur [291]. The occurrence of metabolic disorders during ischemia or tissue hypoxia is relatively well established, but the subsequent reperfusion is the major events leading to cell and tissue dysfunctions [293]. Ischemia–reperfusion injury is the inexplicable aggravation of cellular dysfunction during the restoration of blood flow after a period of ischemia [294]. The reperfusion can lead to potentially very harmful effects, such as necrosis of irreversibly damaged cells, cell swelling, vascular and endothelial injury and mitochondrial dysfunction [295].
Aberrant furin expression in cerebral ischemia
It has been found that the Furin mRNA level in rat hippocampus at 24 h after transient global cerebral ischemia is two-fold of that in sham-operated controls, indicating a possible role furin may play [296]. In a focal ischemic rat model established by middle cerebral artery occlusion, increases in Furin mRNA and protein levels are found in the piriform cortex of the ischemic hemisphere 2 h after reperfusion compared with sham-operated animals, and it is predicted that the elevation of furin may contribute to the disruption of BBB during ischemia [297]. Another recent study found that the level of Furin mRNA in the ipsilateral cortex of hypoxic-ischemic rats had an insignificant increase at 6 h after ischemia, but then decreased significantly at 15 h and was sustained at a low level for 7 days [298], while Furin mRNA in the ipsilateral hippocampus was elevated at 6 h and 3 days but decreased at 15 and 24 h after injury compared with that of the control rats [298]. The change in furin expression is considered to account for the decrease of BDNF in the ipsilateral cortex and hippocampus of the rats [298]. An in vitro study also showed that the protein levels of furin and BDNF are upregulated in cultured rat astrocytes exposed to oxygen–glucose deprivation [64]. These findings indicate that furin may play important roles in the pathogenesis of cerebral ischemia and in the recovery from ischemia brain damage.
Expression of substrates of furin in cerebral ischemia
In addition to the changes in furin expression, the levels of Bdnf mRNA and protein in the ipsilateral cortex and hippocampus of hypoxic-ischemic rats are altered at different degrees at different time points after hypoxic-ischemic injury [298]. Many other studies have also reported changes of MMP levels, including levels of MMP-2, MMP-9 and MMP-14, in the model of focal ischemic rats [297, 299,300,301,302]. In particular, increased expression and activity of MMP-2 and MMP-9 are found in different models of focal cerebral ischemia, implying their potential roles in early matrix degradation, loss of vascular integrity, and neuronal injury in the ischemic lesion [300, 301]. In addition, a significant increase in the cleavage of LRP1 by furin has been found in rats after cerebral ischemia, which is predicted to aggravate neuroinflammation, and administration of a furin inhibitor inhibits the cleavage of LRP1 and decreases co-localization of ICD of LRP1 with furin in ischemic areas [303]. These findings imply that the furin-mediated cleavage of MMPs and LRP1 may be involved in the pathophysiology of ischemic brain injury.
Potential role of furin in the pathology of ischemia
The above observations imply the involvement of furin in the pathology of cerebral ischemia. Changes in furin expression may exist in varied temporal and spatial patterns after ischemic injury in the brain. The upregulation of furin in ischemic patients or animal models may promote the cleavage of MMPs, particularly MMP-2, MMP-9, and MMP-14. The activation of these MMPs leads to early matrix degradation and loss of vascular integrity, and finally contributes to BBB breakdown and neuronal injury in ischemic lesions (Fig. 3c). Moreover, the ICD of LRP1 is increased, which aggravates neuroinflammation. The relationship between changes of furin level and other molecules such as BDNF in ischemic brain injury needs to be elucidated in the future.
Furin in SCZ
SCZ overview
As one of the severe mental diseases, schizophrenia is characterized by cognitive distortions including impairments in concentration, thinking, speed of cognitive information processing, and verbal working memory [304]. These impairments in cognitive functions persist throughout the disease and determine the functional status of patients [305]. The etiology of schizophrenia is complex, commonly associated with genetic variants and changes in development-related factors and regulatory molecules [306].
Aberrant furin expression in SCZ
A study by Fromer et al. in 2016 using RNA sequencing data from the dorsolateral prefrontal cortex of post-mortem SCZ patients identified down-regulation of FURIN transcripts by risk allele [24]. They also found that depletion of furin in zebrafish model has the largest impact on head size, which can be attributed to the furin depletion-induced changes in neural cell proliferation and migration [24]. Furthermore, downregulation of furin expression specifically at the rs4702 G (in the 3' UTR of FURIN) allele by miR-338-3p reduces the production of mBDNF [307]. In addition, the association between pleiotropic effects of FURIN genetic loci and SCZ traits has been reported recently by several different studies [308,309,310]. A study using datasets from the Psychiatric Genomics Consortium related to SCZ, major depressive disorder (MDD) and bipolar disorder (BIP) patients identified rs8039305 in the FURIN gene as a novel pleiotropic locus across the three disorders [309]. Similarly, another study identified rs17514846, a variant within an intron of FURIN gene, as a common trait between SCZ and cardiometabolic disorder [310]. In addition, in C. elegans, the 3'UTR of kpc-1 (furin) promotes dendritic transport and local translation of mRNAs to regulate dendrite branching and self-avoidance [311]. These findings indicate the important role of furin in brain development and in the pathophysiology of SCZ.
Expression of substrates of furin in SCZ
The deregulation of BDNF expression has been extensively studied in SCZ patients and animal models [312,313,314,315,316,317,318,319]. Significant reductions of BDNF mRNA and protein have been observed in the dorsolateral prefrontal cortex of patients with SCZ compared to normal individuals [312]. The reduced BDNF/TrkB signaling in the prefrontal cortex appears to underlie the dysfunctions of inhibitory neurons in subjects with SCZ [313]. Studies have also shown significant reductions of BDNF in the hippocampus as well as NT-3 concentrations in the frontal and parietal cortical areas, in SCZ patients [314]. On the contrary, some studies have shown that the BDNF concentration is significantly increased in cortical areas of post-mortem SCZ patients [314, 315]. In addition, the plasma BDNF levels in schizophrenic patients are remarkably lower than those in the controls, which is predicted to be associated with the decreased hippocampal volume and cognitive impairments in first-episode and chronic SCZ [316, 317]. These findings suggest that the downregulation of neurotrophic factors could be responsible for neural maldevelopment and disturbed neural plasticity in the etiopathogenesis of schizophrenic psychoses. In schizophrenic animal models, reductions of Bdnf mRNA and protein levels have been observed in the cortex and the hippocampus [318, 319]. Decreased serum levels of NGF and NT-3 have been observed in SCZ as well [320,321,322]. In addition to the alterations of neurotrophins, plasma MMP-9 levels are also increased significantly in SCZ patients compared to controls [323], and MMP-9 gene polymorphisms in the brain are found to be associated with SCZ [324, 325]. Besides, increased MMP-2 levels in the CSF of SCZ patients are also reported [326].
Potential role of furin in SCZ pathology
The above findings uncover the involvement of furin in the pathology of SCZ. Furin expression in SCZ patients is downregulated, which in turn affects the maturation of neurotrophins, such as BDNF, NGF and NT3. The chronic low trophic support for neurons leads to neural maldevelopment, dysfunction of inhibitory neurons, disturbed neural plasticity and neurodegeneration, contributing to the impaired cognitive performance/function in SCZ (Fig. 3d). This hypothesis may in part explain the pathogenesis of SCZ. However, the relationships between furin deregulation and changes in MMPs and other furin substrates in SCZ pathology have yet to be investigated.
Furin in depression and anxiety
Currently, there is no report on the changes of furin expression in patients with depression and anxiety. However, the SNP rs8039305 in the FURIN gene has been indicated as a novel pleiotropic locus across the disorders of MDD, BIP and SCZ [309], indicating a potential role of furin in pathological mechanisms of the psychiatric disorders.
Aberrant expression of several substrates of furin has been reported in patients with depression. The serum BDNF level is significantly lower in MMD patients than in healthy controls [327,328,329]. The mBDNF/proBDNF ratio is also decreased [329], suggesting that the reduced BDNF maturation plays a pivotal role in the pathophysiology of MDD. Serum MMP-9 is found to be increased in MDD patients, while MMP-2 is decreased in MDD patients [323, 330], indicating the involvement of MMP-2 and MMP-9 in mood disorders. In addition, MMP-2 levels in the CSF are increased in MDD patients [326], and the state-dependent alterations of MMP-2 and activation of cascades involving MMP-2, MMP-7, and MMP-10 appear to play a role in the pathophysiology of MDD [326]. LRP1 has been reported to be up-regulated in the hippocampus of depressive-like rat model [331].
In anxiety-like disorders, aberrant BDNF expression has also been reported. In the social deprivation stress-triggered anxiety- and depressive-like mice, BDNF levels are reduced in the brain [332]. In serotonin transporter knockout rats with depressive- and anxiety-like behavior, a decrease in mBDNF in the prefrontal cortex has been reported as well [333]. The alterations of proBDNF and mBDNF expression have been indicated in many other diseases with anxiety- and depressive-like behavior [334,335,336,337], highlighting the association between aberrant BDNF expression and anxiety and depression disorders.
Furin-targeting strategies for neurological diseases
Currently, the use of furin-targeting strategies to diagnose or treat neurological disorders has not been reported in clinical studies. However, as described above, furin expression levels are altered in several neurodegenerative and neuropsychiatric diseases; for instance, serum furin level is decreased in AD mice. These highlight the great potential of furin to be a predictive diagnostic marker for neurological disorders in the future.
The potentials of furin-targeting strategies to treat neurological diseases have been suggested in several animal models (Table 2). In AD animal models, injection of Furin-adenovirus into the cortex of Tg2576 mice markedly increases the α-secretase activity of ADAM10 and TACE, which in turn reduces Aβ production [13]. Furin-transgenic mice with brain-specific overexpression of furin exhibit increased dendritic spine density and enhanced learning and memory, which are attributed to the increased mBDNF level caused by furin [26]. In aged APP-C105 mice, treadmill exercise attenuates AD-related symptoms, possibly by ameliorating iron dyshomeostasis and enhancing furin expression, thereby promoting α-secretase-directed processing of APP [23]. Gallic acid treatment in APP/PS1 mice has been shown to increase furin expression, which in turn promotes α-secretase activity and decreases Aβ production, partly reversing the learning and memory impairment in APP/PS1 mice [338]. In addition, cerebrolysin, a peptidergic mixture with neurotrophic-like properties, can improve the survival of neural stem cell grafts and alleviate Aβ deposition in the hippocampus of APP transgenic mice, and this protective effect also involves the activation of furin and increased BDNF expression [339]. On the other hand, knockdown of astrocytic Grin2a in rats reduces furin expression and in turn decreases the maturation and secretion of NGF, aggravating the Aβ-induced memory and cognitive deficits [238]. These findings suggest the potential of increasing furin expression as an effective approach for AD treatment, and open avenues for future targets and strategies for AD prevention and therapeutic interventions.
In paraquat-induced Drosophila model of PD, transgenic knockdown of Fur1 in DA neurons provides significant protection against the loss of DA neurons [265]. In Drosophila models with LRRK2 overexpression, disruption of one allele of Fur1 or postsynaptic knockdown of Fur1 using transgenic RNA interference approach can attenuate the LRRK2-induced retrograde synaptic enhancement [266]. These findings suggest potential involvement of furin in PD pathophysiology and treatment. However, great efforts are urgently needed to explore the role and pharmaceutical potential of furin in PD patients or murine models.
In both KA-induced and PTZ-kindled epileptic mouse models, lentivirus-mediated knockdown of furin in the hippocampus decreases the spontaneous rhythmic electrical activity of cerebral neurons, and suppresses epileptic seizure activity and severity [25]. This protective role is proposed to be associated with the regulation of synaptic transmission by altering the transcription level of postsynaptic gamma-amino butyric acid A receptor [25].
In a global ischemia/reperfusion rat model, monosialoganglioside or flavanol epicatechin treatment both can improve spatial memory retention and acquisition in experimental ischemic rats [340], and these neurotherapeutic effects are found to be related to the increases in furin and NGF levels [340]. In addition, application of furin inhibitor can protect primary cortical neurons from cell death induced by activated NMDA receptors [341], which is possibly attributed to the decrease of furin-mediated cleavage of LRP1 [303]. These findings suggest that manipulating furin expression is potentially a good strategy for the treatment of ischemic brain injury.
In addition, some furin activators and inhibitors have been identified with drug potentials. The small molecules phorbol esters dPPA (12-deoxyphorbol 13-phenylacetate 20-acetate) and dPA (12-deoxyphorbol 13-acetate) exhibit great effects in promoting furin expression via activation of the transcription factor CEBPβ in neuronal cells [34]. On the other hand, polyarginines, such as hexa-D-arginine, significantly inhibit furin activity in vivo [342, 343]. The therapeutic effects of these furin activators and inhibitors in prevention and treatment of neurological disorders need to be investigated further in the future.
Conclusions
A growing body of evidence has suggested the crucial role of furin in the pathophysiological conditions of neurodegenerative and neuropsychiatric diseases. Notably, reduced furin expression is closely associated with the pathogenesis of AD. Pharmaceutical targeting of furin expression has shown great promise for AD treatment. In addition to AD, alterations of furin expression also exist in patients or animal models of epilepsy, cerebral ischemia, or SCZ. Furthermore, changes in the expression of neurotrophins, such as BDNF and NGF, are common to these neurodegenerative and neuropsychiatric diseases, and many are related to the abnormal cleavage of proneurotrophins. In addition to neurotrophins, other substrates of furin such as MMPs and LRP1 also exhibit expression changes in these neurodegenerative and neuropsychiatric diseases. These lines of evidence highlight the important roles of furin and furin-mediated activities in the progression of these diseases, and render furin as a valuable therapeutic target. However, currently very little is known about the cellular and molecular mechanisms of furin regulation in these diseases. Future studies are needed to clarify the molecular mechanisms of furin deregulation and its involvement in the pathogenesis of these diseases, and to develop new diagnostic and treatment strategies.
Availability of data and materials
Available upon request.
Abbreviations
- AD:
-
Alzheimer’s disease
- SCZ:
-
Schizophrenia
- PC:
-
Proprotein convertase
- BDNF:
-
Brain-derived neurotrophic factor
- NGF:
-
Nerve growth factor
- MMPs:
-
Multiple matrix metalloproteases
- PD:
-
Parkinson’s disease
- HIF-1:
-
Hypoxia-inducible factor-1
- TGFβ1:
-
Transforming growth factor beta1
- ER:
-
Endoplasmic reticulum
- DG:
-
Dentate gyrus
- ADAMs:
-
A disintegrin and metalloproteases
- BACE1:
-
Beta-site APP cleaving enzyme 1
- LRP1:
-
Low-density lipoprotein receptor-related protein 1
- GPR37:
-
G-protein-coupled receptor
- mBDNF:
-
Mature BDNF
- TrkB:
-
Tropomyosin-related receptor kinase B
- PI3K:
-
Phosphatidylinositol 3-kinase
- Akt:
-
Protein kinase B
- PLCγ:
-
Phospholipase C-gamma
- CaMK:
-
Calcium-dependent protein kinase
- MAPK:
-
Mitogen-activated protein kinase
- LTP:
-
Long-term potentiation
- p75NTR:
-
P75 neurotrophic receptor
- mNGF:
-
Mature NGF
- TrkA:
-
Tropomyosin-related receptor kinase A
- NT-3:
-
Neurotrophin-3
- NT-4/5:
-
Neurotrophin-4/5
- DA:
-
Dopaminergic
- APP:
-
Amyloid-β precursor protein
- sAPPα:
-
αAPP
- Aβ:
-
Amyloid-β
- CSF:
-
Cerebrospinal fluid
- ICD:
-
Intracellular domain
- NMDA:
-
N-methyl-D-aspartate
- BRI2:
-
Integral membrane protein 2
- NFTs:
-
Neurofibrillary tangles
- BBB:
-
Blood–brain barrier
- TACE:
-
Tumor necrosis factor-α converting enzyme
- LRRK2:
-
Leucine-rich repeat kinase 2
- TLE:
-
Temporal lobe epilepsy
- KA:
-
Kainic acid
- PTZ:
-
Pentylenetetrazol
- MDD:
-
Major depressive disorder
- BIP:
-
Bipolar disorder
References
Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol. 2002;3(10):753–66.
van de Ven WJM, Voorberg J, Fontijn R, Pannekoek H, van den Ouweland AMW, van Duijnhoven HLP, et al. Furin is a subtilisin-like proprotein processing enzyme in higher eukaryotes. Mol Biol Rep. 1990;14(4):265–75.
Wise RJ, Barr PJ, Wong PA, Kiefer MC, Brake AJ, Kaufman RJ. Expression of a human proprotein processing enzyme: correct cleavage of the von Willebrand factor precursor at a paired basic amino acid site. Proc Natl Acad Sci U S A. 1990;87(23):9378–82.
Braun E, Sauter D. Furin-mediated protein processing in infectious diseases and cancer. Clin Transl Immunol. 2019;8(8):e107-e.
Peacock TP, Goldhill DH, Zhou J, Baillon L, Frise R, Swann OC, et al. The furin cleavage site in the SARS-CoV-2 spike protein is required for transmission in ferrets. Nat Microbiol. 2021;6(7):899–909.
Wu Y, Zhao S. Furin cleavage sites naturally occur in coronaviruses. Stem Cell Res. 2020;50: 102115.
**a S, Lan Q, Su S, Wang X, Xu W, Liu Z, et al. The role of furin cleavage site in SARS-CoV-2 spike protein-mediated membrane fusion in the presence or absence of trypsin. Signal Transduct Target Ther. 2020;5(1):92.
Seidah NG, Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat Rev Drug Discovery. 2012;11(5):367–83.
He Z, Khatib AM, Creemers JWM. The proprotein convertase furin in cancer: more than an oncogene. Oncogene. 2022;41(9):1252–62. https://doi.org/10.1038/s41388-021-02175-9.
Jaaks P, Bernasconi M. The proprotein convertase furin in tumour progression. Int J Cancer. 2017;141(4):654–63.
Bresnahan PA, Leduc R, Thomas L, Thorner J, Gibson HL, Brake AJ, et al. Human fur gene encodes a yeast KEX2-like endoprotease that cleaves pro-beta-NGF in vivo. J Cell Biol. 1990;111(6 Pt 2):2851–9.
Meyer A, Chrétien P, Massicotte G, Sargent C, Chrétien M, Marcinkiewicz M. Kainic acid increases the expression of the prohormone convertases furin and PC1 in the mouse hippocampus. Brain Res. 1996;732(1–2):121–32.
Hwang EM, Kim SK, Sohn JH, Lee JY, Kim Y, Kim YS, et al. Furin is an endogenous regulator of α-secretase associated APP processing. Biochem Biophys Res Commun. 2006;349(2):654–9.
Creemers JW, Ines Dominguez D, Plets E, Serneels L, Taylor NA, Multhaup G, et al. Processing of beta-secretase by furin and other members of the proprotein convertase family. J Biol Chem. 2001;276(6):4211–7.
Golubkov VS, Chernov AV, Strongin AY. Intradomain cleavage of inhibitory prodomain is essential to protumorigenic function of membrane type-1 matrix metalloproteinase (MT1-MMP) in vivo. J Biol Chem. 2011;286(39):34215–23.
Wang X, Pei D. Shedding of membrane type matrix metalloproteinase 5 by a furin-type convertase: a potential mechanism for down-regulation. J Biol Chem. 2001;276(38):35953–60.
Mowla SJ, Farhadi HF, Pareek S, Atwal JK, Morris SJ, Seidah NG, et al. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J Biol Chem. 2001;276(16):12660–6.
Thomas E, Joan M, Noel M, Thomas J, Joachim H. The low-density-lipoprotein receptor-related protein (LRP) is processed by furin in vivo and in vitro. Biochem J. 1996;313(1):71–6. https://doi.org/10.1042/bj3130071.
Wang CS, Kavalali ET, Monteggia LM. BDNF signaling in context: From synaptic regulation to psychiatric disorders. Cell. 2022;185(1):62–76.
Gao L, Zhang Y, Sterling K, Song W. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl Neurodegener. 2022;11(1):4.
Camuso S, La Rosa P, Fiorenza MT, Canterini S. Pleiotropic effects of BDNF on the cerebellum and hippocampus: implications for neurodevelopmental disorders. Neurobiol Dis. 2022;163: 105606.
Mohammadi A, Amooeian GV, Rashidi E. Dysfunction in brain-derived neurotrophic factor signaling pathway and susceptibility to schizophrenia, Parkinson’s and Alzheimer’s diseases. Curr Gene Ther. 2018;18(1):45–63.
Choi DH, Kwon KC, Hwang DJ, Koo JH, Um HS, Song HS, et al. Treadmill exercise alleviates brain iron dyshomeostasis accelerating neuronal amyloid-β production, neuronal cell death, and cognitive impairment in transgenic mice model of Alzheimer’s disease. Mol Neurobiol. 2021;58(7):3208–23.
Fromer M, Roussos P, Sieberts SK, Johnson JS, Kavanagh DH, Perumal TM, et al. Gene expression elucidates functional impact of polygenic risk for schizophrenia. Nat Neurosci. 2016;19(11):1442–53.
Yang Y, He M, Tian X, Guo Y, Liu F, Li Y, et al. Transgenic overexpression of furin increases epileptic susceptibility. Cell Death Dis. 2018;9(11):1058.
Zhu B, Zhao L, Luo D, Xu D, Tan T, Dong Z, et al. Furin promotes dendritic morphogenesis and learning and memory in transgenic mice. Cell Mol Life Sci. 2018;75(13):2473–88.
Roebroek AJM, Schalken JA, Bussemakers MJG, van Heerikhuizen H, Onnekink C, Debruyne FMJ, et al. Characterization of human c-fes/fps reveals a new transcription unit (fur) in the immediately upstream region of the proto-oncogene. Mol Biol Rep. 1986;11(2):117–25.
Fuller Robert S, Brake Anthony J, Thorner J. Intracellular targeting and structural conservation of a prohormone-processing endoprotease. Science. 1989;246(4929):482–6.
Rockwell NC, Thorner JW. The kindest cuts of all: crystal structures of Kex2 and furin reveal secrets of precursor processing. Trends Biochem Sci. 2004;29(2):80–7.
Ayoubi TA, Creemers JW, Roebroek AJ, Van de Ven WJ. Expression of the dibasic proprotein processing enzyme furin is directed by multiple promoters. J Biol Chem. 1994;269(12):9298–303.
Lei RX, Shi H, Peng XM, Zhu YH, Cheng J, Chen GH. Influence of a single nucleotide polymorphism in the P1 promoter of the furin gene on transcription activity and hepatitis B virus infection. Hepatology. 2009;50(3):763–71.
Laprise M-H, Grondin F, Cayer P, McDonald PP, Dubois CM. Furin gene (fur) regulation in differentiating human megakaryoblastic Dami cells: involvement of the proximal GATA recognition motif in the P1 promoter and impact on the maturation of furin substrates. Blood. 2002;100(10):3578–87.
Dong X, Chiu H, Park YJ, Zou W, Zou Y, Özkan E, et al. Precise regulation of the guidance receptor DMA-1 by KPC-1/Furin instructs dendritic branching decisions. eLife. 2016. https://doi.org/10.7554/eLife.11008.
Zha JS, Zhu BL, Liu L, Lai YJ, Long Y, Hu XT, et al. Phorbol esters dPPA/dPA promote furin expression involving transcription factor CEBPβ in neuronal cells. Oncotarget. 2017;8(36):60159–72.
Silvestri L, Pagani A, Camaschella C. Furin-mediated release of soluble hemojuvelin: a new link between hypoxia and iron homeostasis. Blood. 2008;111(2):924–31.
Zhou Z, Wang R, Yang X, Lu XY, Zhang Q, Wang YL, et al. The cAMP-responsive element binding protein (CREB) transcription factor regulates furin expression during human trophoblast syncytialization. Placenta. 2014;35(11):907–18.
McMahon S, Grondin F, McDonald PP, Richard DE, Dubois CM. Hypoxia-enhanced expression of the proprotein convertase furin is mediated by hypoxia-inducible factor-1: impact on the bioactivation of proproteins. J Biol Chem. 2005;280(8):6561–9.
Blanchette F, Rudd P, Grondin F, Attisano L, Dubois CM. Involvement of Smads in TGFbeta1-induced furin (fur) transcription. J Cell Physiol. 2001;188(2):264–73.
Ventura E, Weller M, Burghardt I. Cutting edge: ERK1 mediates the autocrine positive feedback loop of TGF-β and furin in glioma-initiating cells. J Immunol. 2017;198(12):4569–74.
Bai L, Chang HM, Zhang L, Zhu YM, Leung PCK. BMP2 increases the production of BDNF through the upregulation of proBDNF and furin expression in human granulosa-lutein cells. FASEB J. 2020;34(12):16129–43.
Osadchuk TV, Shybyryn OV, Kibirev VK. Chemical structure and properties of low-molecular furin inhibitors. Ukr Biochem J. 2016;88(6):5–25.
Gawlik K, Shiryaev SA, Zhu W, Motamedchaboki K, Desjardins R, Day R, et al. Autocatalytic activation of the furin zymogen requires removal of the emerging enzyme’s N-terminus from the active site. PLoS One. 2009;4(4):e5031-e.
Rehemtulla A, Dorner AJ, Kaufman RJ. Regulation of PACE propeptide-processing activity: requirement for a post-endoplasmic reticulum compartment and autoproteolytic activation. Proc Natl Acad Sci U S A. 1992;89(17):8235–9.
Solovyeva NI, Gureeva TA, Timoshenko OS, Moskvitina TA, Kugaevskaya EV. Furin as proprotein convertase and its role in normal and pathological biological processes. Biochem Mosc Suppl B Biomed Chem. 2017;11(2):87–100.
Leduc R, Molloy SS, Thorne BA, Thomas G. Activation of human furin precursor processing endoprotease occurs by an intramolecular autoproteolytic cleavage. J Biol Chem. 1992;267(20):14304–8.
Denault J, Bissonnette L, Longpré J, Charest G, Lavigne P, Leduc R. Ectodomain shedding of furin: kinetics and role of the cysteine-rich region. FEBS Lett. 2002;527(1–3):309–14.
De Bie I, Savaria D, Roebroek AJ, Day R, Lazure C, Van de Ven WJ, et al. Processing specificity and biosynthesis of the Drosophila melanogaster convertases dfurin1, dfurin1-CRR, dfurin1-X, and dfurin2. J Biol Chem. 1995;270(3):1020–8.
Gómez-Saladín E, Luebke AE, Wilson DL, Dickerson IM. Isolation of a cDNA encoding a Kex2-like endoprotease with homology to furin from the nematode Caenorhabditis elegans. DNA Cell Biol. 1997;16(5):663–9.
Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419.
Li D, Liu X, Zhang L, He J, Chen X, Liu S, et al. COVID-19 disease and malignant cancers: the impact for the furin gene expression in susceptibility to SARS-CoV-2. Int J Biol Sci. 2021;17(14):3954–67.
Sjöstedt E, Zhong W, Fagerberg L, Karlsson M, Mitsios N, Adori C, et al. An atlas of the protein-coding genes in the human, pig, and mouse brain. Science. 2020. https://doi.org/10.1126/science.aay5947.
Uhlen M, Zhang C, Lee S, Sjöstedt E, Fagerberg L, Bidkhori G, et al. A pathology atlas of the human cancer transcriptome. Science. 2017. https://doi.org/10.1126/science.aan2507.
Karlsson M, Zhang C, Méar L, Zhong W, Digre A, Katona B, et al. A single-cell type transcriptomics map of human tissues. Sci Adv. 2021. https://doi.org/10.1126/sciadv.abh2169.
Dijk W, Ruppert PMM, Oost LJ, Kersten S. Angiopoietin-like 4 promotes the intracellular cleavage of lipoprotein lipase by PCSK3/furin in adipocytes. J Biol Chem. 2018;293(36):14134–45.
Hosaka M, Nagahama M, Kim WS, Watanabe T, Hatsuzawa K, Ikemizu J, et al. Arg-X-Lys/Arg-Arg motif as a signal for precursor cleavage catalyzed by furin within the constitutive secretory pathway. J Biol Chem. 1991;266(19):12127–30.
Molloy SS, Bresnahan PA, Leppla SH, Klimpel KR, Thomas G. Human furin is a calcium-dependent serine endoprotease that recognizes the sequence Arg-X-X-Arg and efficiently cleaves anthrax toxin protective antigen. J Biol Chem. 1992;267(23):16396–402.
Roebroek AJ, Umans L, Pauli IG, Robertson EJ, van Leuven F, Van de Ven WJ, et al. Failure of ventral closure and axial rotation in embryos lacking the proprotein convertase Furin. Development. 1998;125(24):4863–76.
Liu ZW, Ma Q, Liu J, Li JW, Chen YD. The association between plasma furin and cardiovascular events after acute myocardial infarction. BMC Cardiovasc Disord. 2021;21(1):468.
Wang M, **e Y, Qin D. Proteolytic cleavage of proBDNF to mBDNF in neuropsychiatric and neurodegenerative diseases. Brain Res Bull. 2021;166:172–84.
Farhat D, Ghayad SE, Icard P, Le Romancer M, Hussein N, Lincet H. Lipoic acid-induced oxidative stress abrogates IGF-1R maturation by inhibiting the CREB/furin axis in breast cancer cell lines. Oncogene. 2020;39(17):3604–10.
Ren K, Jiang T, Zheng XL, Zhao GJ. Proprotein convertase furin/PCSK3 and atherosclerosis: new insights and potential therapeutic targets. Atherosclerosis. 2017;262:163–70.
Fernandez C, Rysä J, Almgren P, Nilsson J, Engström G, Orho-Melander M, et al. Plasma levels of the proprotein convertase furin and incidence of diabetes and mortality. J Intern Med. 2018;284(4):377–87.
Zhang Y, Bai X, Zhang Y, Yao S, Cui Y, You LH, et al. Hippocampal iron accumulation impairs synapses and memory via suppressing furin expression and downregulating BDNF maturation. Mol Neurobiol. 2022. https://doi.org/10.1007/s12035-022-02929-w.
Chen Y, Zhang J, Deng M. Furin mediates brain-derived neurotrophic factor upregulation in cultured rat astrocytes exposed to oxygen-glucose deprivation. J Neurosci Res. 2015;93(1):189–94.
Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6(8):603–14.
Yan Q, Rosenfeld RD, Matheson CR, Hawkins N, Lopez OT, Bennett L, et al. Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience. 1997;78(2):431–48.
Leal G, Bramham CR, Duarte CB. BDNF and hippocampal synaptic plasticity. Vitam Horm. 2017;104:153–95.
Foltran RB, Diaz SL. BDNF isoforms: a round trip ticket between neurogenesis and serotonin? J Neurochem. 2016;138(2):204–21.
Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22(3):123–31.
Mizui T, Ishikawa Y, Kumanogoh H, Lume M, Matsumoto T, Hara T, et al. BDNF pro-peptide actions facilitate hippocampal LTD and are altered by the common BDNF polymorphism Val66Met. Proc Natl Acad Sci U S A. 2015;112(23):E3067–74.
Gray K, Ellis V. Activation of pro-BDNF by the pericellular serine protease plasmin. FEBS Lett. 2008;582(6):907–10.
Niculescu D, Michaelsen-Preusse K, Güner Ü, van Dorland R, Wierenga CJ, Lohmann C. A BDNF-mediated push-pull plasticity mechanism for synaptic clustering. Cell Rep. 2018;24(8):2063–74.
Martins CC, Rosa SG, Recchi AMS, Nogueira CW, Zeni G. m-Trifluoromethyl-diphenyl diselenide (m-CF(3)-PhSe)(2) modulates the hippocampal neurotoxic adaptations and abolishes a depressive-like phenotype in a short-term morphine withdrawal in mice. Prog Neuropsychopharmacol Biol Psychiatr. 2020;98: 109803.
Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103(2):211–25.
Blum R, Konnerth A. Neurotrophin-mediated rapid signaling in the central nervous system: mechanisms and functions. Physiol (Bethesda). 2005;20:70–8.
Du Q, Zhu X, Si J. Angelica polysaccharide ameliorates memory impairment in Alzheimer’s disease rat through activating BDNF/TrkB/CREB pathway. Exp Biol Med (Maywood). 2020;245(1):1–10.
Dechant G, Barde YA. The neurotrophin receptor p75(NTR): novel functions and implications for diseases of the nervous system. Nat Neurosci. 2002;5(11):1131–6.
Zagrebelsky M, Korte M. Form follows function: BDNF and its involvement in sculpting the function and structure of synapses. Neuropharmacology. 2014;76:628–38.
Choi SH, Bylykbashi E, Chatila ZK, Lee SW, Pulli B, Clemenson GD, et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science. 2018. https://doi.org/10.1126/science.aan8821.
**e W, Meng X, Zhai Y, Ye T, Zhou P, Nan F, et al. Antidepressant-like effects of the Guanxin Danshen formula via mediation of the CaMK II-CREB-BDNF signalling pathway in chronic unpredictable mild stress-induced depressive rats. Ann Transl Med. 2019;7(20):564.
Girotra P, Behl T, Sehgal A, Singh S, Bungau S. Investigation of the molecular role of brain-derived neurotrophic factor in Alzheimer’s disease. J Mol Neurosci. 2022;72(2):173–86.
Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237(4819):1154–62.
Fahnestock M, Yu G, Coughlin MD. ProNGF: a neurotrophic or an apoptotic molecule? Prog Brain Res. 2004;146:101–10.
Seidah NG, Benjannet S, Pareek S, Savaria D, Hamelin J, Goulet B, et al. Cellular processing of the nerve growth factor precursor by the mammalian pro-protein convertases. Biochem J. 1996;314:951–60.
Yan R, Yalinca H, Paoletti F, Gobbo F, Marchetti L, Kuzmanic A, et al. The structure of the pro-domain of mouse proNGF in contact with the NGF domain. Structure. 2019;27(1):78-89.e3.
Bruno MA, Cuello AC. Activity-dependent release of precursor nerve growth factor, conversion to mature nerve growth factor, and its degradation by a protease cascade. Proc Natl Acad Sci U S A. 2006;103(17):6735–40.
Fahnestock M, Yu G, Michalski B, Mathew S, Colquhoun A, Ross GM, et al. The nerve growth factor precursor proNGF exhibits neurotrophic activity but is less active than mature nerve growth factor. J Neurochem. 2004;89(3):581–92.
Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294(5548):1945–8.
Canu N, Amadoro G, Triaca V, Latina V, Sposato V, Corsetti V, et al. The intersection of NGF/TrkA signaling and amyloid precursor protein processing in Alzheimer’s disease neuropathology. Int J Mol Sci. 2017;18(6):1319.
Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10(3):381–91.
Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, et al. ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron. 2002;36(3):375–86.
Harrington AW, Leiner B, Blechschmitt C, Arevalo JC, Lee R, Mörl K, et al. Secreted proNGF is a pathophysiological death-inducing ligand after adult CNS injury. Proc Natl Acad Sci U S A. 2004;101(16):6226–30.
Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427(6977):843–8.
Fahnestock M, Michalski B, Xu B, Coughlin MD. The precursor pro-nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer’s disease. Mol Cell Neurosci. 2001;18(2):210–20.
Bruno MA, Leon WC, Fragoso G, Mushynski WE, Almazan G, Cuello AC. Amyloid beta-induced nerve growth factor dysmetabolism in Alzheimer disease. J Neuropathol Exp Neurol. 2009;68(8):857–69.
Tiveron C, Fasulo L, Capsoni S, Malerba F, Marinelli S, Paoletti F, et al. ProNGF\NGF imbalance triggers learning and memory deficits, neurodegeneration and spontaneous epileptic-like discharges in transgenic mice. Cell Death Differ. 2013;20(8):1017–30.
Ip NY, Yancopoulos GD. Neurotrophic factors and their receptors. Ann Neurol. 1994;35(Suppl):S13–6.
Barbacid M. Neurotrophic factors and their receptors. Curr Opin Cell Biol. 1995;7(2):148–55.
Pathare-Ingawale P, Chavan-Gautam P. The balance between cell survival and death in the placenta: do neurotrophins have a role? Syst Biol Reprod Med. 2022;68(1):3–12.
Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4(4):299–309.
Keefe KM, Sheikh IS, Smith GM. Targeting neurotrophins to specific populations of neurons: NGF, BDNF, and NT-3 and their relevance for treatment of spinal cord injury. Int J Mol Sci. 2017;18(3):548.
Lai BQ, Bai YR, Han WT, Zhang B, Liu S, Sun JH, et al. Construction of a niche-specific spinal white matter-like tissue to promote directional axon regeneration and myelination for rat spinal cord injury repair. Bioact Mater. 2022;11:15–31.
Ozes B, Moss K, Myers M, Ridgley A, Chen L, Murrey D, Sahenk Z. AAV1.NT-3 gene therapy in a CMT2D model: phenotypic improvements in GarsP278KY/+mice. Brain Commun. 2021. https://doi.org/10.1093/braincomms/fcab252.
Müller ML, Peglau L, Moon LDF, Groß S, Schulze J, Ruhnau J, et al. Neurotrophin-3 attenuates human peripheral blood T cell and monocyte activation status and cytokine production post stroke. Exp Neurol. 2022;347: 113901.
Rodrigues-Amorim D, Iglesias-Martínez-Almeida M, Rivera-Baltanás T, Fernández-Palleiro P, Freiría-Martínez L, Rodríguez-Jamardo C, et al. The role of the second extracellular loop of norepinephrine transporter, neurotrophin-3 and tropomyosin receptor kinase C in T cells: a peripheral biomarker in the etiology of schizophrenia. Int J Mol Sci. 2021;22(16):8499.
Requena-Ocaña N, Araos P, Flores M, García-Marchena N, Silva-Peña D, Aranda J, et al. Evaluation of neurotrophic factors and education level as predictors of cognitive decline in alcohol use disorder. Sci Rep. 2021;11(1):15583.
Ribeiro D, Petrigna L, Pereira FC, Muscella A, Bianco A, Tavares P. The impact of physical exercise on the circulating levels of BDNF and NT 4/5: a review. Int J Mol Sci. 2021;22(16):8814.
Beroun A, Mitra S, Michaluk P, Pijet B, Stefaniuk M, Kaczmarek L. MMPs in learning and memory and neuropsychiatric disorders. Cell Mol Life Sci. 2019;76(16):3207–28.
Ierusalimsky VN, Balaban PM. Type 1 metalloproteinase is selectively expressed in adult rat brain and can be rapidly up-regulated by kainate. Acta Histochem. 2013;115(8):816–26.
Rosenberg GA, Sullivan N, Esiri MM. White matter damage is associated with matrix metalloproteinases in vascular dementia. Stroke. 2001;32(5):1162–8.
Wiera G, Nowak D, van Hove I, Dziegiel P, Moons L, Mozrzymas JW. Mechanisms of NMDA receptor- and voltage-gated L-type calcium channel-dependent hippocampal LTP critically rely on proteolysis that is mediated by distinct metalloproteinases. J Neurosci. 2017;37(5):1240–56.
Vafadari B, Salamian A, Kaczmarek L. MMP-9 in translation: from molecule to brain physiology, pathology, and therapy. J Neurochem. 2016;139(Suppl 2):91–114.
Rosenberg GA. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. 2009;8(2):205–16.
Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, et al. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297(5584):1186–90.
Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516.
Stawowy P, Fleck E. Proprotein convertases furin and PC5: targeting atherosclerosis and restenosis at multiple levels. J Mol Med (Berl). 2005;83(11):865–75.
Cao J, Rehemtulla A, Bahou W, Zucker S. Membrane type matrix metalloproteinase 1 activates pro-gelatinase a without furin cleavage of the N-terminal domain. J Biol Chem. 1996;271(47):30174–80.
Cao J, Rehemtulla A, Pavlaki M, Kozarekar P, Chiarelli C. Furin directly cleaves proMMP-2 in the trans-Golgi network resulting in a nonfunctioning proteinase. J Biol Chem. 2005;280(12):10974–80.
Mittal R, Patel AP, Debs LH, Nguyen D, Patel K, Grati M, et al. Intricate functions of matrix metalloproteinases in physiological and pathological conditions. J Cell Physiol. 2016;231(12):2599–621.
Valente MM, Allen M, Bortolotto V, Lim ST, Conant K, Grilli M. The MMP-1/PAR-1 axis enhances proliferation and neuronal differentiation of adult hippocampal neural progenitor cells. Neural Plast. 2015;2015: 646595.
Allen M, Ghosh S, Ahern GP, Villapol S, Maguire-Zeiss KA, Conant K. Protease induced plasticity: matrix metalloproteinase-1 promotes neurostructural changes through activation of protease activated receptor 1. Sci Rep. 2016;6:35497.
Ogier C, Bernard A, Chollet AM, Diguardher TLE, Hanessian S, Charton G, et al. Matrix metalloproteinase-2 (MMP-2) regulates astrocyte motility in connection with the actin cytoskeleton and integrins. Glia. 2006;54(4):272–84. https://doi.org/10.1002/glia.20349.
Muir EM, Adcock KH, Morgenstern DA, Clayton R, von Stillfried N, Rhodes K, et al. Matrix metalloproteases and their inhibitors are produced by overlap** populations of activated astrocytes. Brain Res Mol Brain Res. 2002;100(1–2):103–17.
Woo MS, Park JS, Choi IY, Kim WK, Kim HS. Inhibition of MMP-3 or -9 suppresses lipopolysaccharide-induced expression of proinflammatory cytokines and iNOS in microglia. J Neurochem. 2008;106(2):770–80.
Bozdagi O, Nagy V, Kwei KT, Huntley GW. In vivo roles for matrix metalloproteinase-9 in mature hippocampal synaptic physiology and plasticity. J Neurophysiol. 2007;98(1):334–44.
Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J, et al. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci. 2006;26(7):1923–34.
Mroczko B, Groblewska M, Zboch M, Kulczyńska A, Koper OM, Szmitkowski M, et al. Concentrations of matrix metalloproteinases and their tissue inhibitors in the cerebrospinal fluid of patients with Alzheimer’s disease. J Alzheimers Dis. 2014;40(2):351–7.
Levin J, Giese A, Boetzel K, Israel L, Högen T, Nübling G, et al. Increased alpha-synuclein aggregation following limited cleavage by certain matrix metalloproteinases. Exp Neurol. 2009;215(1):201–8.
Sung JY, Park SM, Lee C-H, Um JW, Lee HJ, Kim J, et al. Proteolytic cleavage of extracellular secreted α-synuclein via matrix metalloproteinases. J Biol Chem. 2005;280(26):25216–24.
Choi DH, Kim YJ, Kim YG, Joh TH, Beal MF, Kim YS. Role of matrix metalloproteinase 3-mediated alpha-synuclein cleavage in dopaminergic cell death. J Biol Chem. 2011;286(16):14168–77.
Lorenzl S, Albers DS, Narr S, Chirichigno J, Beal MF. Expression of MMP-2, MMP-9, and MMP-1 and their endogenous counterregulators TIMP-1 and TIMP-2 in postmortem brain tissue of Parkinson’s disease. Exp Neurol. 2002;178(1):13–20.
Mullooly M, McGowan PM, Crown J, Duffy MJ. The ADAMs family of proteases as targets for the treatment of cancer. Cancer Biol Ther. 2016;17(8):870–80.
Anders A, Gilbert S, Garten W, Postina R, Fahrenholz F. Regulation of the alpha-secretase ADAM10 by its prodomain and proprotein convertases. FASEB J. 2001;15(10):1837–9.
Seals DF, Courtneidge SA. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev. 2003;17(1):7–30.
Seipold L, Altmeppen H, Koudelka T, Tholey A, Kasparek P, Sedlacek R, et al. In vivo regulation of the a disintegrin and metalloproteinase 10 (ADAM10) by the tetraspanin 15. Cell Mol Life Sci. 2018;75(17):3251–67.
Tousseyn T, Thathiah A, Jorissen E, Raemaekers T, Konietzko U, Reiss K, et al. ADAM10, the rate-limiting protease of regulated intramembrane proteolysis of Notch and other proteins, is processed by ADAMS-9, ADAMS-15, and the gamma-secretase. J Biol Chem. 2009;284(17):11738–47.
Yuan XZ, Sun S, Tan CC, Yu JT, Tan L. The role of ADAM10 in Alzheimer’s disease. J Alzheimers Dis. 2017;58(2):303–22.
Manzine PR, Ettcheto M, Cano A, Busquets O, Marcello E, Pelucchi S, et al. ADAM10 in Alzheimer’s disease: pharmacological modulation by natural compounds and its role as a peripheral marker. Biomed Pharmacother. 2019;113: 108661.
Xu X. γ-Secretase catalyzes sequential cleavages of the AβPP transmembrane domain. J Alzheimers Dis. 2009;16:211–24.
Hong L, Koelsch G, Lin X, Wu S, Terzyan S, Ghosh AK, et al. Structure of the protease domain of memapsin 2 (beta-secretase) complexed with inhibitor. Science. 2000;290(5489):150–3.
Dislich B, Lichtenthaler SF. The membrane-bound aspartyl protease BACE1: molecular and functional properties in Alzheimer’s disease and beyond. Front Physiol. 2012;3:8.
Evin G, Barakat A, Masters CL. BACE: therapeutic target and potential biomarker for Alzheimer’s disease. Int J Biochem Cell Biol. 2010;42(12):1923–6.
Bennett BD, Denis P, Haniu M, Teplow DB, Kahn S, Louis JC, et al. A furin-like convertase mediates propeptide cleavage of BACE, the Alzheimer’s beta -secretase. J Biol Chem. 2000;275(48):37712–7.
Neumann U, Ufer M, Jacobson LH, et al. TheBACE‐1 inhibitor CNP520 for prevention trials in Alzheimer’s disease. EMBO Mol Med. 2018. https://doi.org/10.15252/emmm.201809316.
Hampel H, Shen Y. Beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) as a biological candidate marker of Alzheimer’s disease. Scand J Clin Lab Invest. 2009;69(1):8–12.
Zhong Z, Ewers M, Teipel S, Bürger K, Wallin A, Blennow K, et al. Levels of beta-secretase (BACE1) in cerebrospinal fluid as a predictor of risk in mild cognitive impairment. Arch Gen Psychiatr. 2007;64(6):718–26.
Shen Y, Wang H, Sun Q, Yao H, Keegan AP, Mullan M, et al. Increased plasma beta-secretase 1 may predict conversion to Alzheimer’s disease dementia in individuals with mild cognitive impairment. Biol Psychiatry. 2018;83(5):447–55.
Weiss MM, Williamson T, Babu-Khan S, Bartberger MD, Brown J, Chen K, et al. Design and preparation of a potent series of hydroxyethylamine containing β-secretase inhibitors that demonstrate robust reduction of central β-amyloid. J Med Chem. 2012;55(21):9009–24.
Lahiri DK, Maloney B, Long JM, Greig NH. Lessons from a BACE1 inhibitor trial: off-site but not off base. Alzheimers Dement. 2014;10(5 Suppl):S411–9.
Yan R, Vassar R. Targeting the β secretase BACE1 for Alzheimer’s disease therapy. Lancet Neurol. 2014;13(3):319–29.
Moussa-Pacha NM, Abdin SM, Omar HA, Alniss H, Al-Tel TH. BACE1 inhibitors: current status and future directions in treating Alzheimer’s disease. Med Res Rev. 2020;40(1):339–84.
McDade E, Voytyuk I, Aisen P, Bateman RJ, Carrillo MC, De Strooper B, et al. The case for low-level BACE1 inhibition for the prevention of Alzheimer disease. Nat Rev Neurol. 2021;17(11):703–14.
McKinzie DL, Winneroski LL, Green SJ, Hembre EJ, Erickson JA, Willis BA, et al. Discovery and early clinical development of LY3202626, a low-dose. CNS-penetrant BACE inhibitor J Med Chem. 2021;64(12):8076–100.
Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science. 1995;268(5208):225–32.
Logeat F, Bessia C, Brou C, LeBail O, Jarriault S, Seidah NG, et al. The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc Natl Acad Sci U S A. 1998;95(14):8108–12.
Lathia JD, Mattson MP, Cheng A. Notch: from neural development to neurological disorders. J Neurochem. 2008;107(6):1471–81.
Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7(2):93–102.
Alberi L, Hoey SE, Brai E, Scotti AL, Marathe S. Notch signaling in the brain: in good and bad times. Ageing Res Rev. 2013;12(3):801–14.
Wei Z, Chigurupati S, Arumugam TV, Jo DG, Li H, Chan SL. Notch activation enhances the microglia-mediated inflammatory response associated with focal cerebral ischemia. Stroke. 2011;42(9):2589–94.
Alberi L, Liu S, Wang Y, Badie R, Smith-Hicks C, Wu J, et al. Activity-induced Notch signaling in neurons requires Arc/Arg3.1 and is essential for synaptic plasticity in hippocampal networks. Neuron. 2011;69(3):437–44.
Arumugam TV, Baik SH, Balaganapathy P, Sobey CG, Mattson MP, Jo DG. Notch signaling and neuronal death in stroke. Prog Neurobiol. 2018;165–167:103–16.
Sargin D, Botly LC, Higgs G, Marsolais A, Frankland PW, Egan SE, et al. Disrupting Jagged1-Notch signaling impairs spatial memory formation in adult mice. Neurobiol Learn Mem. 2013;103:39–49.
Sha L, Wu X, Yao Y, Wen B, Feng J, Sha Z, et al. Notch signaling activation promotes seizure activity in temporal lobe epilepsy. Mol Neurobiol. 2014;49(2):633–44.
Wolf BB, Lopes MB, VandenBerg SR, Gonias SL. Characterization and immunohistochemical localization of alpha 2-macroglobulin receptor (low-density lipoprotein receptor-related protein) in human brain. Am J Pathol. 1992;141(1):37–42.
Herz J. The LDL receptor gene family: (un)expected signal transducers in the brain. Neuron. 2001;29(3):571–81.
Lin JP, Mironova YA, Shrager P, Giger RJ. LRP1 regulates peroxisome biogenesis and cholesterol homeostasis in oligodendrocytes and is required for proper CNS myelin development and repair. eLife. 2017. https://doi.org/10.7554/eLife.30498.
Zlokovic BV, Deane R, Sagare AP, Bell RD, Winkler EA. Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer’s amyloid β-peptide elimination from the brain. J Neurochem. 2010;115(5):1077–89.
Maier W, Bednorz M, Meister S, Roebroek A, Weggen S, Schmitt U, et al. LRP1 is critical for the surface distribution and internalization of the NR2B NMDA receptor subtype. Mol Neurodegener. 2013;8:25.
Nakajima C, Kulik A, Frotscher M, Herz J, Schäfer M, Bock HH, et al. Low density lipoprotein receptor-related protein 1 (LRP1) modulates N-methyl-D-aspartate (NMDA) receptor-dependent intracellular signaling and NMDA-induced regulation of postsynaptic protein complexes. J Biol Chem. 2013;288(30):21909–23.
Zurhove K, Nakajima C, Herz J, Bock HH, May P. γ-Secretase limits the inflammatory response through the processing of LRP1. Sci Signal. 2008. https://doi.org/10.1126/scisignal.1164263.
Shinohara M, Tachibana M, Kanekiyo T, Bu G. Role of LRP1 in the pathogenesis of Alzheimer’s disease: evidence from clinical and preclinical studies. J Lipid Res. 2017;58(7):1267–81.
May P, Rohlmann A, Bock HH, Zurhove K, Marth JD, Schomburg ED, et al. Neuronal LRP1 functionally associates with postsynaptic proteins and is required for normal motor function in mice. Mol Cell Biol. 2004;24(20):8872–83.
Gan M, Jiang P, McLean P, Kanekiyo T, Bu G. Low-density lipoprotein receptor-related protein 1 (LRP1) regulates the stability and function of GluA1 α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor in neurons. PLoS ONE. 2014;9(12): e113237.
Liu Q, Trotter J, Zhang J, Peters MM, Cheng H, Bao J, et al. Neuronal LRP1 knockout in adult mice leads to impaired brain lipid metabolism and progressive, age-dependent synapse loss and neurodegeneration. J Neurosci. 2010;30(50):17068–78.
Liu CC, Hu J, Tsai CW, Yue M, Melrose HL, Kanekiyo T, et al. Neuronal LRP1 regulates glucose metabolism and insulin signaling in the brain. J Neurosci. 2015;35(14):5851–9.
Romeo R, Boden-El Mourabit D, Scheller A, Mark MD, Faissner A. Low-density lipoprotein receptor-related protein 1 (LRP1) as a novel regulator of early astroglial differentiation. Front Cell Neurosci. 2021;15: 642521.
Safina D, Schlitt F, Romeo R, Pflanzner T, Pietrzik CU, Narayanaswami V, et al. Low-density lipoprotein receptor-related protein 1 is a novel modulator of radial glia stem cell proliferation, survival, and differentiation. Glia. 2016;64(8):1363–80.
Auderset L, Pitman KA, Cullen CL, Pepper RE, Taylor BV, Foa L, et al. Low-density lipoprotein receptor-related protein 1 (LRP1) is a negative regulator of oligodendrocyte progenitor cell differentiation in the adult mouse brain. Front Cell Dev Biol. 2020;8: 564351.
Mattila SO, Tuhkanen HE, Lackman JJ, Konzack A, Morató X, Argerich J, et al. GPR37 is processed in the N-terminal ectodomain by ADAM10 and furin. FASEB J. 2021;35(6): e21654.
Imai Y, Soda M, Inoue H, Hattori N, Mizuno Y, Takahashi R. An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell. 2001;105(7):891–902.
Murakami T, Shoji M, Imai Y, Inoue H, Kawarabayashi T, Matsubara E, et al. Pael-R is accumulated in Lewy bodies of Parkinson’s disease. Ann Neurol. 2004;55(3):439–42.
Morató X, Garcia-Esparcia P, Argerich J, Llorens F, Zerr I, Paslawski W, et al. Ecto-GPR37: a potential biomarker for Parkinson’s disease. Transl Neurodegener. 2021;10(1):8.
Marazziti D, Mandillo S, Di Pietro C, Golini E, Matteoni R, Tocchini-Valentini GP. GPR37 associates with the dopamine transporter to modulate dopamine uptake and behavioral responses to dopaminergic drugs. Proc Natl Acad Sci U S A. 2007;104(23):9846–51.
Lopes JP, Morató X, Souza C, Pinhal C, Machado NJ, Canas PM, et al. The role of parkinson’s disease-associated receptor GPR37 in the hippocampus: functional interplay with the adenosinergic system. J Neurochem. 2015;134(1):135–46.
Batiuk MY, Martirosyan A, Wahis J, de Vin F, Marneffe C, Kusserow C, et al. Identification of region-specific astrocyte subtypes at single cell resolution. Nat Commun. 2020;11(1):1220.
Yang HJ, Vainshtein A, Maik-Rachline G, Peles E. G protein-coupled receptor 37 is a negative regulator of oligodendrocyte differentiation and myelination. Nat Commun. 2016;7:10884.
Dusonchet J, Bensadoun JC, Schneider BL, Aebischer P. Targeted overexpression of the parkin substrate Pael-R in the nigrostriatal system of adult rats to model Parkinson’s disease. Neurobiol Dis. 2009;35(1):32–41.
Marazziti D, Golini E, Mandillo S, Magrelli A, Witke W, Matteoni R, et al. Altered dopamine signaling and MPTP resistance in mice lacking the Parkinson’s disease-associated GPR37/parkin-associated endothelin-like receptor. Proc Natl Acad Sci U S A. 2004;101(27):10189–94.
Zhang X, Mantas I, Fridjonsdottir E, Andrén PE, Chergui K, Svenningsson P. Deficits in motor performance, neurotransmitters and synaptic plasticity in elderly and experimental Parkinsonian mice lacking GPR37. Front Aging Neurosci. 2020;12:84.
Mandillo S, Golini E, Marazziti D, Di Pietro C, Matteoni R, Tocchini-Valentini GP. Mice lacking the Parkinson’s related GPR37/PAEL receptor show non-motor behavioral phenotypes: age and gender effect. Genes Brain Behav. 2013;12(4):465–77.
Veenit V, Zhang X, Ambrosini A, Sousa V, Svenningsson P. The effect of early life stress on emotional behaviors in GPR37KO mice. Int J Mol Sci. 2021;23(1):410. https://doi.org/10.3390/ijms23010410.
Eggert S, Thomas C, Kins S, Hermey G. Trafficking in Alzheimer’s disease: modulation of APP transport and processing by the transmembrane proteins LRP1, SorLA, SorCS1c, Sortilin, and Calsyntenin. Mol Neurobiol. 2018;55(7):5809–29.
Eggert S, Kins S, Endres K, Brigadski T. Brothers in arms: proBDNF/BDNF and sAPPα/Aβ-signaling and their common interplay with ADAM10, TrkB, p75NTR, sortilin, and sorLA in the progression of Alzheimer’s disease. Biol Chem. 2022;403(1):43–71.
Yang M, Virassamy B, Vijayaraj SL, Lim Y, Saadipour K, Wang YJ, et al. The intracellular domain of sortilin interacts with amyloid precursor protein and regulates its lysosomal and lipid raft trafficking. PLoS ONE. 2013;8(5): e63049.
Takamura A, Sato Y, Watabe D, Okamoto Y, Nakata T, Kawarabayashi T, et al. Sortilin is required for toxic action of Aβ oligomers (AβOs): extracellular AβOs trigger apoptosis, and intraneuronal AβOs impair degradation pathways. Life Sci. 2012;91(23–24):1177–86.
Jansen P, Giehl K, Nyengaard JR, Teng K, Lioubinski O, Sjoegaard SS, et al. Roles for the pro-neurotrophin receptor sortilin in neuronal development, aging and brain injury. Nat Neurosci. 2007;10(11):1449–57.
Lewin GR, Nykjaer A. Pro-neurotrophins, sortilin, and nociception. Eur J Neurosci. 2014;39(3):363–74.
Vaegter CB, Jansen P, Fjorback AW, Glerup S, Skeldal S, Kjolby M, et al. Sortilin associates with Trk receptors to enhance anterograde transport and neurotrophin signaling. Nat Neurosci. 2011;14(1):54–61.
Finan GM, Okada H, Kim TW. BACE1 retrograde trafficking is uniquely regulated by the cytoplasmic domain of sortilin. J Biol Chem. 2011;286(14):12602–16.
Mazella J, Borsotto M, Heurteaux C. The involvement of Sortilin/NTSR3 in depression as the progenitor of spadin and its role in the membrane expression of TREK-1. Front Pharmacol. 2018;9:1541.
Pittois K, Deleersnijder W, Merregaert J. cDNA sequence analysis, chromosomal assignment and expression pattern of the gene coding for integral membrane protein 2B. Gene. 1998;217(1–2):141–9.
Kim SH, Wang R, Gordon DJ, Bass J, Steiner DF, Thinakaran G, et al. Familial British dementia: expression and metabolism of BRI. Ann N Y Acad Sci. 2000;920:93–9.
Kim SH, Creemers JW, Chu S, Thinakaran G, Sisodia SS. Proteolytic processing of familial British dementia-associated BRI variants: evidence for enhanced intracellular accumulation of amyloidogenic peptides. J Biol Chem. 2002;277(3):1872–7.
Kim SH, Wang R, Gordon DJ, Bass J, Steiner DF, Lynn DG, et al. Furin mediates enhanced production of fibrillogenic ABri peptides in familial British dementia. Nat Neurosci. 1999;2(11):984–8.
Knight SD, Presto J, Linse S, Johansson J. The BRICHOS domain, amyloid fibril formation, and their relationship. Biochemistry. 2013;52(43):7523–31.
Poska H, Haslbeck M, Kurudenkandy FR, Hermansson E, Chen G, Kostallas G, et al. Dementia-related Bri2 BRICHOS is a versatile molecular chaperone that efficiently inhibits Aβ42 toxicity in Drosophila. Biochem J. 2016;473(20):3683–704.
Akiyama H, Kondo H, Arai T, Ikeda K, Kato M, Iseki E, et al. Expression of BRI, the normal precursor of the amyloid protein of familial British dementia, in human brain. Acta Neuropathol. 2004;107(1):53–8.
Willander H, Presto J, Askarieh G, Biverstål H, Frohm B, Knight SD, et al. BRICHOS domains efficiently delay fibrillation of amyloid β-peptide. J Biol Chem. 2012;287(37):31608–17.
Peng S, Fitzen M, Jörnvall H, Johansson J. The extracellular domain of Bri2 (ITM2B) binds the ABri peptide (1–23) and amyloid beta-peptide (Abeta1-40): Implications for Bri2 effects on processing of amyloid precursor protein and Abeta aggregation. Biochem Biophys Res Commun. 2010;393(3):356–61.
Vidal R, Frangione B, Rostagno A, Mead S, Révész T, Plant G, et al. A stop-codon mutation in the BRI gene associated with familial British dementia. Nature. 1999;399(6738):776–81.
Matsuda S, Senda T. BRI2 as an anti-Alzheimer gene. Med Mol Morphol. 2019;52(1):1–7.
Del Campo M, Hoozemans JJ, Dekkers LL, Rozemuller AJ, Korth C, Müller-Schiffmann A, et al. BRI2-BRICHOS is increased in human amyloid plaques in early stages of Alzheimer’s disease. Neurobiol Aging. 2014;35(7):1596–604.
Holthuis JC, Jansen EJ, Schoonderwoert VT, Burbach JP, Martens GJ. Biosynthesis of the vacuolar H+-ATPase accessory subunit Ac45 in Xenopus pituitary. Eur J Biochem. 1999;262(2):484–91.
Abbas YM, Wu D, Bueler SA, Robinson CV, Rubinstein JL. Structure of V-ATPase from the mammalian brain. Science. 2020;367(6483):1240–6.
Wang R, Long T, Hassan A, Wang J, Sun Y, **e XS, et al. Cryo-EM structures of intact V-ATPase from bovine brain. Nat Commun. 2020;11(1):3921.
Louagie E, Taylor NA, Flamez D, Roebroek AJ, Bright NA, Meulemans S, et al. Role of furin in granular acidification in the endocrine pancreas: identification of the V-ATPase subunit Ac45 as a candidate substrate. Proc Natl Acad Sci U S A. 2008;105(34):12319–24.
Brouwers B, Coppola I, Vints K, Dislich B, Jouvet N, Van Lommel L, et al. Loss of furin in β-cells induces an mTORC1-ATF4 anabolic pathway that leads to β-cell dysfunction. Diabetes. 2021;70(2):492–503.
Supek F, Supekova L, Mandiyan S, Pan YC, Nelson H, Nelson N. A novel accessory subunit for vacuolar H(+)-ATPase from chromaffin granules. J Biol Chem. 1994;269(39):24102–6.
Hodi FS, Schmollinger JC, Soiffer RJ, Salgia R, Lynch T, Ritz J, et al. ATP6S1 elicits potent humoral responses associated with immune-mediated tumor destruction. Proc Natl Acad Sci U S A. 2002;99(10):6919–24.
Feng H, Cheng T, Pavlos NJ, Yip KH, Carrello A, Seeber R, et al. Cytoplasmic terminus of vacuolar type proton pump accessory subunit Ac45 is required for proper interaction with V(0) domain subunits and efficient osteoclastic bone resorption. J Biol Chem. 2008;283(19):13194–204.
Dulac A, Issa AR, Sun J, Matassi G, Jonas C, Chérif-Zahar B, et al. A novel neuron-specific regulator of the V-ATPase in Drosophila. eNeuro. 2021
Jansen EJ, Timal S, Ryan M, Ashikov A, van Scherpenzeel M, Graham LA, et al. ATP6AP1 deficiency causes an immunodeficiency with hepatopathy, cognitive impairment and abnormal protein glycosylation. Nat Commun. 2016;7:11600.
Vitória JJ, Trigo D. Revisiting APP secretases: an overview on the holistic effects of retinoic acid receptor stimulation in APP processing. Cel Mol Life Sci. 2022;79(2):1–5.
Jan AT, Azam M, Rahman S, Almigeiti AMS, Choi DH, Lee EJ, et al. Perspective insights into disease progression, diagnostics, and therapeutic approaches in Alzheimer’s disease: a judicious update. Front Aging Neurosci. 2017;9:356.
Cummings J, Lee G, Ritter A, Sabbagh M, Zhong K. Alzheimer’s disease drug development pipeline: 2020. Alzheimers Dement (N Y). 2020;6(1): e12050.
Goedert M, Spillantini MG. A century of Alzheimer’s disease. Science. 2006;314(5800):777–81.
Zhang Y, Song W. Islet amyloid polypeptide: another key molecule in Alzheimer’s pathogenesis? Prog Neurobiol. 2017;153:100–20.
Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–6.
Yang Y, Wang JZ. Nature of Tau-associated neurodegeneration and the molecular mechanisms. J Alzheimers Dis. 2018;62(3):1305–17.
Erickson MA, Banks WA. Blood-brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J Cereb Blood Flow Metab. 2013;33(10):1500–13.
James SA, Churches QI, de Jonge MD, Birchall IE, Streltsov V, McColl G, et al. Iron, copper, and zinc concentration in Aβ plaques in the APP/PS1 mouse model of Alzheimer’s disease correlates with metal levels in the surrounding neuropil. ACS Chem Neurosci. 2017;8(3):629–37.
Peters DG, Connor JR, Meadowcroft MD. The relationship between iron dyshomeostasis and amyloidogenesis in Alzheimer’s disease: two sides of the same coin. Neurobiol Dis. 2015;81:49–65.
Aisen PS, Davis KL. Inflammatory mechanisms in Alzheimer’s disease: implications for therapy. Am J Psychiatry. 1994;151(8):1105–13.
Swerdlow RH, Khan SM. A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Med Hypoth. 2004;63(1):8–20.
Liu Z, Zhou T, Ziegler AC, Dimitrion P, Zuo L. Oxidative stress in neurodegenerative diseases: from molecular mechanisms to clinical applications. Oxid Med Cell Longev. 2017;2017:2525967.
Neth BJ, Craft S. Insulin resistance and Alzheimer’s disease: bioenergetic linkages. Front Aging Neurosci. 2017;9:345.
Guan C, Dang R, Cui Y, Liu L, Chen X, Wang X, et al. Characterization of plasma metal profiles in Alzheimer’s disease using multivariate statistical analysis. PLoS ONE. 2017;12(7): e0178271.
Du Z, Song Y, Chen X, Zhang W, Zhang G, Li H, et al. Knockdown of astrocytic Grin2a aggravates β-amyloid-induced memory and cognitive deficits through regulating nerve growth factor. Aging Cell. 2021;20(8): e13437.
Song Y, Du Z, Chen X, Zhang W, Zhang G, Li H, et al. Astrocytic N-methyl-D-aspartate receptors protect the hippocampal neurons against amyloid-β142-induced synaptotoxicity by regulating nerve growth factor. J Alzheimers Dis. 2022;85(1):167–78.
Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron. 1991;7(5):695–702.
Connor B, Young D, Yan Q, Faull RL, Synek B, Dragunow M. Brain-derived neurotrophic factor is reduced in Alzheimer’s disease. Brain Res Mol Brain Res. 1997;49(1–2):71–81.
Holsinger RM, Schnarr J, Henry P, Castelo VT, Fahnestock M. Quantitation of BDNF mRNA in human parietal cortex by competitive reverse transcription-polymerase chain reaction: decreased levels in Alzheimer’s disease. Brain Res Mol Brain Res. 2000;76(2):347–54.
Garzon D, Yu G, Fahnestock M. A new brain-derived neurotrophic factor transcript and decrease in brain-derived neurotrophic factor transcripts 1, 2 and 3 in Alzheimer’s disease parietal cortex. J Neurochem. 2002;82(5):1058–64.
Ferrer I, Marín C, Rey MJ, Ribalta T, Goutan E, Blanco R, et al. BDNF and full-length and truncated TrkB expression in Alzheimer disease. Implications in therapeutic strategies. J Neuropathol Exp Neurol. 1999;58(7):729–39.
Michalski B, Fahnestock M. Pro-brain-derived neurotrophic factor is decreased in parietal cortex in Alzheimer’s disease. Brain Res Mol Brain Res. 2003;111(1–2):148–54.
Peng S, Wuu J, Mufson EJ, Fahnestock M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer’s disease. J Neurochem. 2005;93(6):1412–21.
Gerenu G, Martisova E, Ferrero H, Carracedo M, Rantamäki T, Ramirez MJ, et al. Modulation of BDNF cleavage by plasminogen-activator inhibitor-1 contributes to Alzheimer’s neuropathology and cognitive deficits. Biochim Biophys Acta Mol Basis Dis. 2017;1863(4):991–1001.
Kimura R, Devi L, Ohno M. Partial reduction of BACE1 improves synaptic plasticity, recent and remote memories in Alzheimer’s disease transgenic mice. J Neurochem. 2010;113(1):248–61.
Kaminari A, Giannakas N, Tzinia A, Tsilibary EC. Overexpression of matrix metalloproteinase-9 (MMP-9) rescues insulin-mediated impairment in the 5XFAD model of Alzheimer’s disease. Sci Rep. 2017;7(1):683.
Peng S, Wuu J, Mufson EJ, Fahnestock M. Increased proNGF levels in subjects with mild cognitive impairment and mild Alzheimer disease. J Neuropathol Exp Neurol. 2004;63(6):641–9.
Mufson EJ, He B, Nadeem M, Perez SE, Counts SE, Leurgans S, et al. Hippocampal proNGF signaling pathways and β-amyloid levels in mild cognitive impairment and Alzheimer disease. J Neuropathol Exp Neurol. 2012;71(11):1018–29.
Nagarsheth MH, Viehman A, Lippa SM, Lippa CF. Notch-1 immunoexpression is increased in Alzheimer’s and Pick’s disease. J Neurol Sci. 2006;244(1–2):111–6.
Holsinger RMD, McLean CA, Beyreuther K, Masters CL, Evin G. Increased expression of the amyloid precursor β-secretase in Alzheimer’s disease. Ann Neurol. 2002;51(6):783–6.
Li R, Lindholm K, Yang L-B, Yue X, Citron M, Yan R, et al. Amyloid β peptide load is correlated with increased β-secretase activity in sporadic Alzheimer’s disease patients. Proc Natl Acad Sci U S A. 2004;101(10):3632–7.
Leake A, Morris CM, Whateley J. Brain matrix metalloproteinase 1 levels are elevated in Alzheimer’s disease. Neurosci Lett. 2000;291(3):201–3.
Py NA, Bonnet AE, Bernard A, Marchalant Y, Charrat E, Checler F, et al. Differential spatio-temporal regulation of MMPs in the 5xFAD mouse model of Alzheimer’s disease: evidence for a pro-amyloidogenic role of MT1-MMP. Front Aging Neurosci. 2014;6:247.
Fleitas C, Piñol-Ripoll G, Marfull P, Rocandio D, Ferrer I, Rampon C, et al. proBDNF is modified by advanced glycation end products in Alzheimer’s disease and causes neuronal apoptosis by inducing p75 neurotrophin receptor processing. Mol Brain. 2018;11(1):68.
Arélin K, Kinoshita A, Whelan CM, Irizarry MC, Rebeck GW, Strickland DK, et al. LRP and senile plaques in Alzheimer’s disease: colocalization with apolipoprotein E and with activated astrocytes. Brain Res Mol Brain Res. 2002;104(1):38–46.
Matsui T, Ingelsson M, Fukumoto H, Ramasamy K, Kowa H, Frosch MP, et al. Expression of APP pathway mRNAs and proteins in Alzheimer’s disease. Brain Res. 2007;1161:116–23.
Kang DE, Pietrzik CU, Baum L, Chevallier N, Merriam DE, Kounnas MZ, et al. Modulation of amyloid beta-protein clearance and Alzheimer’s disease susceptibility by the LDL receptor-related protein pathway. J Clin Invest. 2000;106(9):1159–66.
Shang J, Yamashita T, Tian F, Li X, Liu X, Shi X, et al. Chronic cerebral hypoperfusion alters amyloid-β transport related proteins in the cortical blood vessels of Alzheimer’s disease model mouse. Brain Res. 2019;1723: 146379.
Tofaris GK. Initiation and progression of α-synuclein pathology in Parkinson’s disease. Cell Mol Life Sci. 2022;79(4):210.
Lotharius J, Brundin P. Pathogenesis of Parkinson’s disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci. 2002;3(12):932–42.
Zhang J. Mining imaging and clinical data with machine learning approaches for the diagnosis and early detection of Parkinson’s disease. NPJ Parkinson’s disease. 2022;8(1):13.
Maksoud E, Liao EH, Haghighi AP. A neuron-glial trans-signaling cascade mediates LRRK2-induced neurodegeneration. Cell Rep. 2019;26(7):1774-86.e4.
Penney J, Tsurudome K, Liao EH, Kauwe G, Gray L, Yanagiya A, et al. LRRK2 regulates retrograde synaptic compensation at the Drosophila neuromuscular junction. Nat Commun. 2016;7:12188.
Salehi Z, Mashayekhi F. Brain-derived neurotrophic factor concentrations in the cerebrospinal fluid of patients with Parkinson’s disease. J Clin Neurosci. 2009;16(1):90–3.
Scalzo P, Kümmer A, Bretas TL, Cardoso F, Teixeira AL. Serum levels of brain-derived neurotrophic factor correlate with motor impairment in Parkinson’s disease. J Neurol. 2010;257(4):540–5.
Wang Y, Liu H, Zhang BS, Soares JC, Zhang XY. Low BDNF is associated with cognitive impairments in patients with Parkinson’s disease. Parkinsonism Relat Disord. 2016;29:66–71.
Gupta V, Singh MK, Garg RK, Pant KK, Khattri S. Evaluation of peripheral matrix metalloproteinase-1 in Parkinson’s disease: a case-control study. Int J Neurosci. 2014;124(2):88–92.
Thijs RD, Ryvlin P, Surges R. Autonomic manifestations of epilepsy: emerging pathways to sudden death? Nat Rev Neurol. 2021;17(12):774–88.
Casillas-Espinosa PM, Powell KL, O’Brien TJ. Regulators of synaptic transmission: roles in the pathogenesis and treatment of epilepsy. Epilepsia. 2012;53(Suppl 9):41–58.
Manford M. Recent advances in epilepsy. J Neurol. 2017;264(8):1811–24.
Zsurka G, Kunz WS. Mitochondrial dysfunction and seizures: the neuronal energy crisis. Lancet Neurol. 2015;14(9):956–66.
Conboy K, Henshall DC, Brennan GP. Epigenetic principles underlying epileptogenesis and epilepsy syndromes. Neurobiol Dis. 2021;148: 105179.
Mathern GW, Babb TL, Micevych PE, Blanco CE, Pretorius JK. Granule cell mRNA levels for BDNF, NGF, and NT-3 correlate with neuron losses or supragranular mossy fiber sprouting in the chronically damaged and epileptic human hippocampus. Mol Chem Neuropathol. 1997;30(1–2):53–76.
Takahashi M, Hayashi S, Kakita A, Wakabayashi K, Fukuda M, Kameyama S, et al. Patients with temporal lobe epilepsy show an increase in brain-derived neurotrophic factor protein and its correlation with neuropeptide Y. Brain Res. 1999;818(2):579–82.
Thomas AX, Cruz Del Angel Y, Gonzalez MI, Carrel AJ, Carlsen J, Lam PM, et al. Rapid increases in proBDNF after pilocarpine-induced status epilepticus in mice are associated with reduced proBDNF cleavage machinery. eNeuro. 2016;3(1):452.
Gall CM, Isackson PJ. Limbic seizures increase neuronal production of messenger RNA for nerve growth factor. Science. 1989;245(4919):758–61.
Gall C, Murray K, Isackson PJ. Kainic acid-induced seizures stimulate increased expression of nerve growth factor mRNA in rat hippocampus. Brain Res Mol Brain Res. 1991;9(1–2):113–23.
Mehrabi S, Janahamdi M, Joghataie MT, Barati M, Marzban M, Hadjighassem M, et al. Blockade of p75 neurotrophin receptor reverses irritability and anxiety-related behaviors in a rat model of status epilepticus. Iran Biomed J. 2018;22(4):264–74.
Heinrich C, Lähteinen S, Suzuki F, Anne-Marie L, Huber S, Häussler U, et al. Increase in BDNF-mediated TrkB signaling promotes epileptogenesis in a mouse model of mesial temporal lobe epilepsy. Neurobiol Dis. 2011;42(1):35–47.
Wang R, Zeng GQ, Tong RZ, Zhou D, Hong Z. Serum matrix metalloproteinase-2: A potential biomarker for diagnosis of epilepsy. Epilepsy Res. 2016;122:114–9.
Wang R, Zeng GQ, Liu X, Tong RZ, Zhou D, Hong Z. Evaluation of serum matrix metalloproteinase-3 as a biomarker for diagnosis of epilepsy. J Neurol Sci. 2016;367:291–7.
Broekaart DW, Bertran A, Jia S, Korotkov A, Senkov O, Bongaarts A, et al. The matrix metalloproteinase inhibitor IPR-179 has antiseizure and antiepileptogenic effects. J Clin Invest. 2021. https://doi.org/10.1172/JCI138332.
Konopka A, Grajkowska W, Ziemiańska K, Roszkowski M, Daszkiewicz P, Rysz A, et al. Matrix metalloproteinase-9 (MMP-9) in human intractable epilepsy caused by focal cortical dysplasia. Epilepsy Res. 2013;104(1–2):45–58.
Mizoguchi H, Nakade J, Tachibana M, Ibi D, Someya E, Koike H, et al. Matrix metalloproteinase-9 contributes to kindled seizure development in pentylenetetrazole-treated mice by converting pro-BDNF to mature BDNF in the hippocampus. J Neurosci. 2011;31(36):12963–71.
Kim GW, Kim HJ, Cho KJ, Kim HW, Cho YJ, Lee BI. The role of MMP-9 in integrin-mediated hippocampal cell death after pilocarpine-induced status epilepticus. Neurobiol Dis. 2009;36(1):169–80.
Gorter JA, Van Vliet EA, Rauwerda H, Breit T, Stad R, van Schaik L, et al. Dynamic changes of proteases and protease inhibitors revealed by microarray analysis in CA3 and entorhinal cortex during epileptogenesis in the rat. Epilepsia. 2007;48(Suppl 5):53–64.
Korotkov A, Broekaart DWM, van Schep**en J, Anink JJ, Baayen JC, Idema S, et al. Increased expression of matrix metalloproteinase 3 can be attenuated by inhibition of microRNA-155 in cultured human astrocytes. J Neuroinflammation. 2018;15(1):211.
Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371(9624):1612–23.
Campbell BCV, Khatri P. Stroke. Lancet. 2020;396(10244):129–42.
Soares ROS, Losada DM, Jordani MC, Évora P, Castro ESO. Ischemia/reperfusion injury revisited: an overview of the latest pharmacological strategies. Int J Mol Sci. 2019;20(20):5034.
Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190(3):255–66.
Evora PR, Pearson PJ, Seccombe JF, Schaff HV. Ischemia-reperfusion lesion Physiopathologic aspects and the importance of the endothelial function. Arq Bras Cardiol. 1996;66(4):239–45.
Yokota N, Uchijima M, Nishizawa S, Namba H, Koide Y. Identification of differentially expressed genes in rat hippocampus after transient global cerebral ischemia using subtractive cDNA cloning based on polymerase chain reaction. Stroke. 2001;32(1):168–74.
Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27(4):697–709.
**ong LL, Chen J, Du RL, Liu J, Chen YJ, Hawwas MA, et al. Brain-derived neurotrophic factor and its related enzymes and receptors play important roles after hypoxic-ischemic brain damage. Neural Regen Res. 2021;16(8):1453–9.
Planas AM, Solé S, Justicia C. Expression and activation of matrix metalloproteinase-2 and -9 in rat brain after transient focal cerebral ischemia. Neurobiol Dis. 2001;8(5):834–46.
Chang DI, Hosomi N, Lucero J, Heo JH, Abumiya T, Mazar AP, et al. Activation systems for latent matrix metalloproteinase-2 are upregulated immediately after focal cerebral ischemia. J Cereb Blood Flow Metab. 2003;23(12):1408–19.
Heo JH, Lucero J, Abumiya T, Koziol JA, Copeland BR, del Zoppo GJ. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19(6):624–33.
Gasche Y, Fujimura M, Morita-Fujimura Y, Copin JC, Kawase M, Massengale J, et al. Early appearance of activated matrix metalloproteinase-9 after focal cerebral ischemia in mice: a possible role in blood-brain barrier dysfunction. J Cereb Blood Flow Metab. 1999;19(9):1020–8.
Yamada M, Hayashi H, Suzuki K, Sato S, Inoue D, Iwatani Y, et al. Furin-mediated cleavage of LRP1 and increase in ICD of LRP1 after cerebral ischemia and after exposure of cultured neurons to NMDA. Sci Rep. 2019;9(1):11782.
Morozova A, Zorkina Y, Abramova O, Pavlova O, Pavlov K, Soloveva K, et al. Neurobiological highlights of cognitive impairment in psychiatric disorders. Int J Mol Sci. 2022;23(3):1217.
Xu MY, Wong AHC. GABAergic inhibitory neurons as therapeutic targets for cognitive impairment in schizophrenia. Acta Pharmacol Sin. 2018;39(5):733–53.
Geschwind DH, Flint J. Genetics and genomics of psychiatric disease. Science. 2015;349(6255):1489–94.
Hou Y, Liang W, Zhang J, Li Q, Ou H, Wang Z, et al. Schizophrenia-associated rs4702 G allele-specific downregulation of FURIN expression by miR-338-3p reduces BDNF production. Schizophr Res. 2018;199:176–80.
Korologou-Linden R, Leyden GM, Relton CL, Richmond RC, Richardson TG. Multi-omics analyses of cognitive traits and psychiatric disorders highlights brain-dependent mechanisms. Hum Mol Genet. 2021. https://doi.org/10.1093/hmg/ddab016.
Wang H, Yi Z, Shi T. Novel loci and potential mechanisms of major depressive disorder, bipolar disorder, and schizophrenia. Sci China Life Sci. 2022;65(1):167–83.
Perry BI, Bowker N, Burgess S, Wareham NJ, Upthegrove R, Jones PB, et al. Evidence for shared genetic aetiology between schizophrenia, cardiometabolic, and inflammation-related traits: genetic correlation and colocalization analyses. Schizophr Bull Open. 2022;3(1):sgac001.
Zou Y, Shih M, Chiu H, Ferreira T, Suzuki N, Zou W, et al. The kpc-1 (furin) 3’ UTR promotes dendritic transport and local translation of mRNAs to regulate dendrite branching and self-avoidance of a nociceptive neuron. bioRxiv. 2021. https://doi.org/10.1101/2021.08.03.453128.
Weickert CS, Hyde TM, Lipska BK, Herman MM, Weinberger DR, Kleinman JE. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2003;8(6):592–610.
Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN, et al. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci. 2005;25(2):372–83.
Durany N, Michel T, Zöchling R, Boissl KW, Cruz-Sánchez FF, Riederer P, et al. Brain-derived neurotrophic factor and neurotrophin 3 in schizophrenic psychoses. Schizophr Res. 2001;52(1–2):79–86.
Takahashi M, Shirakawa O, Toyooka K, Kitamura N, Hashimoto T, Maeda K, et al. Abnormal expression of brain-derived neurotrophic factor and its receptor in the corticolimbic system of schizophrenic patients. Mol Psychiatry. 2000;5(3):293–300.
Yang Y, Liu Y, Wang G, Hei G, Wang X, Li R, et al. Brain-derived neurotrophic factor is associated with cognitive impairments in first-episode and chronic schizophrenia. Psychiatry Res. 2019;273:528–36.
Rizos EN, Papathanasiou M, Michalopoulou PG, Mazioti A, Douzenis A, Kastania A, et al. Association of serum BDNF levels with hippocampal volumes in first psychotic episode drug-naive schizophrenic patients. Schizophr Res. 2011;129(2–3):201–4.
Ashe PC, Chlan-Fourney J, Juorio AV, Li XM. Brain-derived neurotrophic factor (BDNF) mRNA in rats with neonatal ibotenic acid lesions of the ventral hippocampus. Brain Res. 2002;956(1):126–35.
Yuan Q, Yang F, **ao Y, Tan S, Husain N, Ren M, et al. Regulation of brain-derived neurotrophic factor exocytosis and gamma-aminobutyric acidergic interneuron synapse by the schizophrenia susceptibility gene dysbindin-1. Biol Psychiatry. 2016;80(4):312–22.
Dai N, Jie H, Duan Y, **ong P, Xu X, Chen P, et al. Different serum protein factor levels in first-episode drug-naive patients with schizophrenia characterized by positive and negative symptoms. Psychiatr Clin Neurosci. 2020;74(9):472–9.
Qin XY, Wu HT, Cao C, Loh YP, Cheng Y. A meta-analysis of peripheral blood nerve growth factor levels in patients with schizophrenia. Mol Psychiatry. 2017;22(9):1306–12.
Vargas HE, Gama CS, Andreazza AC, Medeiros D, Stertz L, Fries G, et al. Decreased serum neurotrophin 3 in chronically medicated schizophrenic males. Neurosci Lett. 2008;440(3):197–201.
Domenici E, Willé DR, Tozzi F, Prokopenko I, Miller S, McKeown A, et al. Plasma protein biomarkers for depression and schizophrenia by multi analyte profiling of case-control collections. PLoS ONE. 2010;5(2): e9166.
Lepeta K, Purzycka KJ, Pachulska-Wieczorek K, Mitjans M, Begemann M, Vafadari B, et al. A normal genetic variation modulates synaptic MMP-9 protein levels and the severity of schizophrenia symptoms. EMBO Mol Med. 2017;9(8):1100–16.
Vafadari B, Mitra S, Stefaniuk M, Kaczmarek L. Psychosocial stress induces schizophrenia-like behavior in mice with reduced MMP-9 activity. Front Behav Neurosci. 2019;13:195.
Omori W, Hattori K, Kajitani N, Tsuchioka MO, Boku S, Kunugi H, et al. Increased matrix metalloproteinases in cerebrospinal fluids of patients with major depressive disorder and schizophrenia. Int J Neuropsychopharmacol. 2020;23(11):713–20.
Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry. 2003;54(1):70–5.
Caldieraro MA, McKee M, Leistner-Segal S, Vares EA, Kubaski F, Spanemberg L, et al. Val66Met polymorphism association with serum BDNF and inflammatory biomarkers in major depression. World J Biol Psychiatry. 2018;19(5):402–9.
Jiang H, Chen S, Li C, Lu N, Yue Y, Yin Y, et al. The serum protein levels of the tPA-BDNF pathway are implicated in depression and antidepressant treatment. Transl Psychiatry. 2017;7(4): e1079.
Shibasaki C, Takebayashi M, Itagaki K, Abe H, Kajitani N, Okada-Tsuchioka M, et al. Altered serum levels of matrix metalloproteinase-2, -9 in response to electroconvulsive therapy for mood disorders. Int J Neuropsychopharmacol. 2016;19(9):pyw019.
Wang H, **ao L, Wang H, Wang G. Involvement of chronic unpredictable mild stress-induced hippocampal LRP1 up-regulation in microtubule instability and depressive-like behavior in a depressive-like adult male rat model. Physiol Behav. 2020;215: 112749.
Berry A, Bellisario V, Capoccia S, Tirassa P, Calza A, Alleva E, et al. Social deprivation stress is a triggering factor for the emergence of anxiety- and depression-like behaviours and leads to reduced brain BDNF levels in C57BL/6J mice. Psychoneuroendocrinology. 2012;37(6):762–72.
Sbrini G, Brivio P, Bosch K, Homberg JR, Calabrese F. Enrichment environment positively influences depression- and anxiety-like behavior in serotonin transporter knockout rats through the modulation of neuroplasticity, spine, and GABAergic markers. Genes. 2020;11(11):1248.
Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10(9):1089–93.
Zhang Y, Bi YX, Chen J, Li S, Wu XX, Zhang L, et al. Association of Peroxiredoxin 1 and brain-derived neurotrophic factor serum levels with depression and anxiety symptoms in patients with irritable bowel syndrome. Gen Hosp Psychiatry. 2021;68:59–64.
Malheiros RT, Delgado HO, Felber DT, Kraus SI, Dos Santos ARS, Manfredini V, et al. Mood disorders are associated with the reduction of brain derived neurotrophic factor in the hypocampus in rats submitted to the hipercaloric diet. Metab Brain Dis. 2021;36(1):145–51.
Aksoy H, Ergun T, Akkiprik M, Peker Eyuboglu İ, Seckin Gencosmanoglu D, Cöbek Ünalan GP, et al. The impact of antipsoriatic treatment on serum pro-BDNF, BDNF levels, depression, anxiety scores, and quality of life. Dermatol Ther. 2021;34(2): e14872.
Mori T, Koyama N, Yokoo T, Segawa T, Maeda M, Sawmiller D, et al. Gallic acid is a dual α/β-secretase modulator that reverses cognitive impairment and remediates pathology in Alzheimer mice. J Biol Chem. 2020;295(48):16251–66.
Rockenstein E, Desplats P, Ubhi K, Mante M, Florio J, Adame A, et al. Neuro-peptide treatment with Cerebrolysin improves the survival of neural stem cell grafts in an APP transgenic model of Alzheimer disease. Stem Cell Res. 2015;15(1):54–67.
Choucry AM, Al-Shorbagy MY, Attia AS, El-Abhar HS. Pharmacological manipulation of Trk, p75NTR, and NGF balance restores memory deficit in global ischemia/reperfusion model in rats. J Mol Neurosci. 2019;68(1):78–90.
Yamada M, Hayashi H, Yuuki M, Matsushima N, Yuan B, Takagi N. Furin inhibitor protects against neuronal cell death induced by activated NMDA receptors. Sci Rep. 2018;8(1):5212.
Cameron A, Appel J, Houghten RA, Lindberg I. Polyarginines are potent furin inhibitors. J Biol Chem. 2000;275(47):36741–9.
Sarac MS, Cameron A, Lindberg I. The furin inhibitor hexa-D-arginine blocks the activation of Pseudomonas aeruginosa exotoxin A in vivo. Infect Immun. 2002;70(12):7136–9.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 32170979 and 32070962), the Science and Technology Project of Hebei Education Department (grant number ZD2021327), and the Natural Science Foundation of Hebei Normal University (grant number L2021Z04).
Author information
Authors and Affiliations
Contributions
YZC and GG designed the work. YZ, XG and GG wrote a major portion of the manuscript and made all the figures. XB and SY wrote a small portion of the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, Y., Gao, X., Bai, X. et al. The emerging role of furin in neurodegenerative and neuropsychiatric diseases. Transl Neurodegener 11, 39 (2022). https://doi.org/10.1186/s40035-022-00313-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40035-022-00313-1