Abstract
Background
Chimeric antigen receptor T-cell (CAR-T) therapy is increasingly used in patients with refractory haematological malignancies but can induce severe adverse events. We aimed to describe the clinical features and outcomes of patients admitted to the intensive care unit (ICU) after CAR-T therapy.
Methods
This retrospective observational cohort study included consecutive adults admitted to either of two French ICUs in 2018–2022 within 3 months after CAR-T therapy.
Results
Among 238 patients given CAR-T therapy, 84 (35.3%) required ICU admission and were included in the study, a median of 5 [0–7] days after CAR-T infusion. Median SOFA and SAPSII scores were 3 [2–6] and 39 [30–48], respectively. Criteria for cytokine release syndrome were met in 80/84 (95.2%) patients, including 18/80 (22.5%) with grade 3–4 toxicity. Immune effector cell-associated neurotoxicity syndrome (ICANS) occurred in 46/84 (54.8%) patients, including 29/46 (63%) with grade 3–4 toxicity. Haemophagocytic lymphohistiocytosis was diagnosed in 15/84 (17.9%) patients. Tocilizumab was used in 73/84 (86.9%) patients, with a median of 2 [1–4] doses. Steroids were given to 55/84 (65.5%) patients, including 21/55 (38.2%) given high-dose pulse therapy. Overall, 23/84 (27.4%) patients had bacterial infections, 3/84 (3.6%) had fungal infections (1 invasive pulmonary aspergillosis and 2 Mucorales), and 2 (2.4%) had cytomegalovirus infection. Vasopressors were required in 23/84 (27.4%), invasive mechanical ventilation in 12/84 (14.3%), and dialysis in 4/84 (4.8%) patients. Four patients died in the ICU (including 2 after ICU readmission, i.e., overall mortality was 4.8% of patients). One year after CAR-T therapy, 41/84 (48.9%) patients were alive and in complete remission, 14/84 (16.7%) were alive and in relapse, and 29/84 (34.5%) had died. These outcomes were similar to those of patients never admitted to the ICU.
Conclusion
ICU admission is common after CAR-T therapy and is usually performed to manage specific toxicities. Our experience is encouraging, with low ICU mortality despite a high rate of grade 3–4 toxicities, and half of patients being alive and in complete remission at one year.
Similar content being viewed by others
Background
Chimeric antigen receptor T-cell (CAR-T) therapy is an innovative approach for managing refractory haematological malignancies. Autologous cytotoxic T lymphocytes are genetically modified to specifically recognise a tumour antigen, thereby causing tumour lysis [1, 2]. CAR-T therapy, first approved by the Food and Drug Administration in 2017 and by the European Medicines Agency in 2018, has provided extended survival and complete remission in patients with relapsed or refractory diffuse large B-cell lymphoma [3, 4] or acute lymphoblastic leukaemia (ALL) [5]. With newer indications such as multiple myeloma (MM) [6, 7], earlier treatment in lymphoma [8], and ongoing clinical trials in patients with solid tumours [9, 10], the number of patients given CAR-T therapy is predicted to increase steadily.
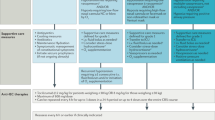
However, the inflammatory response generated by activated CAR-T cells can lead to potentially life-threatening complications, namely, cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). CRS is the most frequent, develo** in up to 93% patients [3,4,5], and is defined as a febrile capillary leak syndrome, with hypotension, hypoxia, and organ failures in severe cases [19,20,21]. All our patients with ICANS also had CRS, in accordance with current pathophysiological knowledge [32, 33], although in the international CARTTAS cohort, 7/238 patients had ICANS without CRS [19]. The treatment of CRS relies on the IL-6 receptor antagonist tocilizumab and on corticosteroids as a second-line treatment, whereas corticosteroids are the recommended first-line treatment for ICANS [30, 31]. All ICU studies found similar rates of tocilizumab and corticosteroids use, but with major differences across centres regarding the drugs chosen, doses, timing of administration and discontinuation, and additional treatments [19,20,21]. Although guidelines have been issued, current recommendations rely on expert opinion and observational data, as no randomised controlled trials are available. Concern has been expressed regarding potential deleterious effects of high-dose corticosteroids on the efficacy of CAR-T therapy [20, 34], as the overwhelming majority of deaths after CAR-T infusion are due to disease progression or relapse [19, 34, 35]. Further studies are therefore needed to define the optimal management of CRS and ICANS.
HLH developed in 17.9% of our patients, compared to 3.8%–5% in previous reports [19, 20]. This discrepancy may be related to differences in the underlying malignancies, type of CAR-T used, and diagnostic criteria for HLH. This syndrome is challenging to distinguish from CRS and sepsis in clinical practice [30]. A recent study emphasised the need for further research to better recognize, define, and treat HLH in CAR-T recipients [36].
As ICU admission usually occurs early after CAR-T therapy, during the neutropenic phase, sepsis is a major concern and the main differential diagnosis of CRS in daily practice. The CARTTAS study found bacterial infection to be independently associated with a twofold higher mortality rate [19]. Microbiologically documented sepsis has been reported to occur in 16% to 30% of patients, in kee** with the 27% proportion in our cohort [19,20,21]. Three of our patients had fungal infections. The optimal prophylactic or pre-emptive strategy for infection after CAR-T therapy has yet to be determined but as of now, urgent broad-spectrum antibiotics in all febrile patients with neutropenia remain essential [37, 38].
Finally, although severe CRS and ICANS may require life-supporting interventions, recovery usually occurs within a week. ICU admission after CAR-T therapy was consistently associated with greater than 90% survival at ICU discharge [19,20,21]. Our experience was similar, with an overall mortality rate of 4.8%. Nonetheless, the subset of patients who required ICU readmission had a higher mortality. Importantly, ICU survivors had similar one-year outcomes to those of patients not admitted to the ICU.
Study implications
Our findings of excellent in-ICU and one-year outcomes, despite a substantial rate of high-grade toxicities following CAR-T therapy, support early unrestricted ICU admission without undue concern about a possible negative impact of critical care on CAR-T efficacy and the haematological prognosis. Moreover, our results imply that because sepsis is common and indistinguishable from CRS in clinical practice, sepsis can never be ruled out during the neutropenic phase and should be thoroughly investigated, and broad-spectrum antibiotics administered without delay. Finally, despite rapid advances in pathophysiological understanding, the main immunomodulatory treatments and specific therapies used to control CAR-T toxicities remain largely empirical. The prospective collection of data in nationwide registries would increase the amount of available data, thereby providing a strong basis for further research.
Strengths and limitations
Our study has several limitations. First, the difficulty of distinguishing CRS from sepsis carries a risk of adjudication bias, particularly given the retrospective design. Similarly, confounding factors such as uraemia, high fever, and use of beta-lactams or neurotropic agents may lead to ICANS-like symptoms. However, in the absence of a reference-standard diagnostic strategy, we strictly applied ASTCT grading recommendations for CRS and ICANS and reported microbiologically documented infections, refraining from classifying patients based on subjective criteria. Second, we included patients over a nearly 4-year period, during which both critical-care and haematology teams gained experience in managing CAR-T therapy recipients. The criteria for transferring these patients to the ICU may therefore have changed over the recruitment period. Our results may not apply to other ICUs with different admission policies. However, our study adds relevant and comprehensive data from two experienced ICUs that were among the first to treat CAR-T therapy recipients in France. Third, the study design prevented us from evaluating how the specific treatments used in the ICU may have affected patient outcomes. However, current recommendations rely chiefly on low-level observational evidence. Fourth, more than 70% of patients were treated with axicabtagen ciloleucel, and our findings may not apply to patients treated with other CAR products. Finally, the very low mortality precluded a multivariable analysis designed to identify independent predictors of death.
Conclusion
In conclusion, our study confirms that intensive care is an integral part of the management of patients given CAR-T therapy. Both specific toxicities (CRS, ICANS, and HLH) and sepsis may require intensive care. The short-term outcomes are excellent, and critical care is not associated with worse one-year haematological outcomes. Studies are needed to investigate the interplay between CAR-T efficacy, toxicity, and the impact of immunomodulating treatments. Moreover, as the current standard of care remains largely empirical, interventional studies are now needed to guide clinical practice.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CAR-T:
-
Chimeric antigen receptor T-cell
- CRS:
-
Cytokine release syndrome
- HLH:
-
Haemophagocytic lymphohistiocytosis
- ICANS:
-
Immune effector cell-associated neurotoxicity syndrome
- ICU:
-
Intensive care unit
References
Bachmann M. The UniCAR system: a modular CAR T cell approach to improve the safety of CAR T cells. Immunol Lett. 2019;211:13–22.
McGuirk J, Waller EK, Qayed M, Abhyankar S, Ericson S, Holman P, et al. Building blocks for institutional preparation of CTL019 delivery. Cytotherapy. 2017;19(9):1015–24.
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-Cell lymphoma. N Engl J Med. 2019;380(1):45–56.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory Large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–44.
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–48.
Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380(18):1726–37.
Munshi NC, Anderson LD, Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705–16.
Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640–54.
Lou JX. An Exploratory Study of αPD1-MSLN-CAR T Cells Secreting PD-1 Nanobodies for the Treatment of MSLN-positive Advanced Solid Tumors. 2022. Available from: https://clinicaltrials.gov/study/NCT05373147. Accesed 9 Aug 2023.
Fang W. A Phase I clinical study of CD70-targeting CAR-T therapy in the treatment of CD70-positive advanced/metastatic solid tumors. 2022. Available from: https://clinicaltrials.gov/study/NCT05518253. Accesed 9 Aug 2023.
** Z, **ang R, Qing K, Li X, Zhang Y, Wang L, et al. The severe cytokine release syndrome in phase I trials of CD19-CAR-T cell therapy: a systematic review. Ann Hematol. 2018;97(8):1327–35.
Chou CK, Turtle CJ. Insight into mechanisms associated with cytokine release syndrome and neurotoxicity after CD19 CAR-T cell immunotherapy. Bone Marrow Transplant. 2019;54(Suppl 2):780–4.
Hay KA. Cytokine release syndrome and neurotoxicity after CD19 chimeric antigen receptor-modified (CAR-) T cell therapy. Br J Haematol. 2018;183(3):364–74.
Santomasso BD, Park JH, Salloum D, Riviere I, Flynn J, Mead E, et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 2018;8(8):958–71.
Nastoupil LJ, Jain MD, Feng L, Spiegel JY, Ghobadi A, Lin Y, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US lymphoma CAR T consortium. J Clin Oncol Off J Am Soc Clin Oncol. 2020;38(27):3119–28.
June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379(1):64–73.
Hill JA, Li D, Hay KA, Green ML, Cherian S, Chen X, et al. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood. 2018;131(1):121–30.
Darmon M, Bourmaud A, Georges Q, Soares M, Jeon K, Oeyen S, et al. Changes in critically ill cancer patients’ short-term outcome over the last decades: results of systematic review with meta-analysis on individual data. Intensive Care Med. 2019;45(7):977–87.
Azoulay É, Castro P, Maamar A, Metaxa V, de Moraes AG, Voigt L, et al. Outcomes in patients treated with chimeric antigen receptor T-cell therapy who were admitted to intensive care (CARTTAS): an international, multicentre, observational cohort study. Lancet Haematol. 2021;8(5):e355–64.
Gutierrez C, Brown ART, May HP, Beitinjaneh A, Stephens RS, Rajendram P, et al. Critically ill patients treated for chimeric antigen receptor-related toxicity: a multicenter study. Crit Care Med. 2022;50(1):81–92.
Valade S, Darmon M, Zafrani L, Mariotte E, Lemiale V, Bredin S, et al. The use of ICU resources in CAR-T cell recipients: a hospital-wide study. Ann Intensive Care. 2022;12(1):75.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–8.
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–38.
Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third International consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10.
Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–63.
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the european society of intensive care medicine. Intensiv Care Med. 1996;22(7):707–10.
Yakoub-Agha I, Moreau AS, Ahmad I, Borel C, Hadhoum N, Masouridi-Levrat S, et al. Prise en charge pratique du syndrome de relargage des cytokines (CRS) post-CAR-T cells chez l’adulte et l’enfant : recommandation de la Société francophone de greffe de moelle et de thérapie cellulaire (SFGM-TC). Bull Cancer. 2019;106(1, Supplement):S102–9.
Cornillon J, Hadhoum N, Roth-Guepin G, Quessar A, Platon L, Ouachée-Chardin M, et al. Prise en charge pratique d’une encéphalopathie liée au traitement par cellules CAR-T chez l’adulte et l’enfant : recommandations de la Société francophone de greffe de moelle et de thérapie cellulaire (SFGM-TC). Bull Cancer. 2020;107(1, Supplement):S12–7 ([In French]).
Tudesq JJ, Yakoub-Agha M, Bay JO, Courbon C, Paul F, Picard M, et al. Management of cytokine release syndrome and macrophage activation syndrome following CAR-T cell therapy: guidelines from the SFGM-TC. Bull Cancer (Paris). 2023;110(2S):S116–22.
Picard M, Sterin A, Bay JO, Courbon C, Moreau AS, Paul F, et al. Management of neurotoxicity following CAR-T cell therapy: recommendations of the SFGM-TC. Bull Cancer (Paris). 2023;110(2S):S123–31.
Rubin DB, Al Jarrah A, Li K, LaRose S, Monk AD, Ali AB, et al. Clinical predictors of neurotoxicity after chimeric antigen receptor T-cell therapy. JAMA Neurol. 2020;77(12):1536–42.
Morris EC, Neelapu SS, Giavridis T, Sadelain M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol. 2022;22(2):85–96.
Strati P, Ahmed S, Furqan F, Fayad LE, Lee HJ, Iyer SP, et al. Prognostic impact of corticosteroids on efficacy of chimeric antigen receptor T-cell therapy in large B-cell lymphoma. Blood. 2021;137(23):3272–6.
Lemoine J, Bachy E, Cartron G, Beauvais D, Gastinne T, Di Blasi R, et al. Nonrelapse mortality after CAR T-cell therapy for large B-cell lymphoma: a LYSA study from the DESCAR-T registry. Blood Adv. 2023;7(21):6589–98.
Lichtenstein DA, Schischlik F, Shao L, Steinberg SM, Yates B, Wang HW, et al. Characterization of HLH-like manifestations as a CRS variant in patients receiving CD22 CAR T cells. Blood. 2021;138(24):2469–84.
Paul F, Vicente C, Courbon C, Moreau AS, Picard M, Pochon C, et al. Prevention and management of infections in patients undergoing CAR T-cell therapy: recommendations of the francophone society of bone marrow transplantation and cellular therapy (SFGM-TC). Bull Cancer (Paris). 2021;108(12S):S90–7.
Haroon A, Muhsen IN, Abid MB, Albabtain A, Alahmari A, Ahmed SO, et al. Infectious complications and preventative strategies following chimeric antigen receptor T-cells (CAR-T cells) therapy for B-cell malignancies. Hematol Oncol Stem Cell Ther. 2022;15(3):153–8.
Acknowledgements
None.
Funding
No part of the work received any financial support from any source.
Author information
Authors and Affiliations
Contributions
CLC and EC designed the study. CLC, AC, CS, and QQ collected the data. CLC and EC performed the statistical analysis, interpreted the data, and drafted the manuscript. MP helped build the survival analysis model and the Venn diagram. All authors contributed to recruit patients. All authors revised the manuscript for important intellectual content. All authors read and approved the final submitted manuscript. All authors have agreed to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of the French Intensive Care Society (CE SRLF 22-044) on July 7, 2022. In accordance with French law on retrospective studies of anonymized healthcare data, informed consent was not required.
Consent for publication
Not applicable.
Competing interests
EC has received lecturer and conference-speaker fees, as well as reimbursements of travel and accommodation expenses related to attending scientific meetings, from Gilead, Shionogi, and Sanofi-Genzyme. JBL has received lecturer and conference-speaker fees from BD and Zoll. RH reports honoraria from Kite/Gilead, Novartis, Incyte, Janssen, MSD, Takeda and Roche; and the receipt of consultancy fees from Kite/Gilead, Novartis, Bristol-Myers Squibb/Celgene, ADC Therapeutics, Incyte, and Miltenyi Biotec. None of the other authors have relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Appendix 1: STROBE Statement. Appendix 2: Supplementary methods. Appendix 3: American Society for Transplantation and Cellular Therapy (ASTCT) grading for CRS and ICANS. Appendix 4: Encephalopathy Assessment Tools for Grading of ICANS: CARTOX-10 and ICE scores. Appendix 5: Study flowchart. Appendix 6: Infectious complications during the 97 ICU stays in 84 patients. Appendix 7: Additional data on patients with haemophagocytic lymphohistiocytosis (HLH) (n=15). Appendix 8: Use of second-line immunosuppressors. Appendix 9: Additional data on patients with repeated admissions. Appendix 10: Causes of death in CAR-T recipients
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Le Cacheux, C., Couturier, A., Sortais, C. et al. Features and outcomes of patients admitted to the ICU for chimeric antigen receptor T cell-related toxicity: a French multicentre cohort. Ann. Intensive Care 14, 20 (2024). https://doi.org/10.1186/s13613-024-01247-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-024-01247-9