Abstract
Background
Recent progress in the induced generation of dopaminergic (DA) neurons from different types of stem cells or reprogrammed somatic cells holds tremendous potential for the treatment of Parkinson’s disease (PD). However, the lack of a reliable source for cell replacement therapy remains a major limitation in the treatment of human neurological disorders. Additionally, the current protocols for in vitro differentiation or cell reprogramming to generate human DA neurons are laborious, time-consuming, and expensive, and efficient conversion of human spermatogonial stem cells (hSSCs) to functional DA neurons has not yet been achieved.
Methods
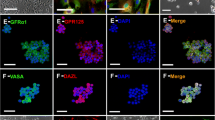
Primary hSSCs from testicular tissues of patients were exposed to an improved induction system, which consisted mainly of olfactory ensheathing cell conditioned culture medium (OECCM) and a set of defined cell-extrinsic factors and small molecules. Morphological changes were assessed, along with the expression of various DA neuron phenotypic markers (e.g., Tuj-1, TH, Nurr1, DAT) and several critical pro-DA neurogenesis effectors (e.g., EN-1, Pitx3, Foxa2, Lmx1a, Lmx1b, and OTX2). In addition, transcriptome analysis was used to further evaluate the genetic similarity between the artificially differentiated DA neurons and genuine ones. Concomitantly, the functional properties of converted DA neurons including synapse formation, dopamine release, electrophysiological activity, and neuron-specific Ca2+ signaling images were determined. Finally, hSSCs in the early stage of induction were evaluated for survival, differentiation, migration, tumorigenicity in the mouse striatum, and improvement of functional deficits in MPTP-induced PD animals.
Results
The hSSC-derived neurons not only acquired neuronal morphological features but also expressed various phenotypic genes and protein characteristic of DA neurons and several effectors critical for pro-DA neurogenesis. Strikingly, as the period of induction was prolonged, expression of the critical molecules for DA neuron epigenetic status gradually increased while hSSC-specific markers sharply decreased. After 3 weeks of induction, the transdifferentiation efficiency reached 21%. In addition, hierarchical clustering analysis showed that the differentiated DA neurons closely resembled genuine ones. Furthermore, the hSSC-derived neurons gained sophisticated functional properties of wild-type DA neurons, and pro-induced hSSCs efficiently survived, migrated, and differentiated into DA neurons without tumorigenesis after transplantation into mouse striatum, leading to improvement of functional deficits in PD animals.
Conclusions
The results showed that, using the present improved straightforward approach, hSSCs could acquire DA neuron morphological features and functional properties and rescue parkinsonian phenotypes. Our strategy for the conversion of hSSCs into DA neurons is very efficient and thus may provide an alternative approach suitable for clinical cell therapy to treat neurodegenerative diseases including PD.
Similar content being viewed by others
Background
Parkinson’s disease (PD) is the second most prevalent neurodegenerative disorders characterized by marked depletion of dopamine caused by the loss or degeneration of DA neurons in the substantia nigra (SN) of the midbrain [1,2,3]. Thus far, there is still no curative treatment for PD although existing treatments such as deep brain stimulation and pharmacotherapies (including levodopa monoamine oxidase B) can alleviate some of the symptoms; they tend to lose efficacy over time and thus do not prevent irreversible disabilities indefinitely [4,5,6,7]. Based on the molecular pathological properties of PD, cell replenishment therapy, especially for DA neurons, is considered the most attractive approach to treating PD patients. The advent of stem cells and cellular reprogramming has brought the gospel for PD patients, opening the way for potential applications of cell-based therapy. Among various types of cells, neural stem cells (NSCs) have long been demonstrated to be a promising candidate for treating PD. Nevertheless, the clinical application of NSCs is still challenging due to limited cell sourcing and ethical concerns. Therefore, other types of stem cells that are readily available appear to hold enormous promise for cell replacement therapy in regenerative diseases.
In various studies, reprogrammed somatic cells or stem cells including induced pluripotent stem cells (iPSCs), embryonic stem cells (ESCs), and mesenchymal stem cells (MSCs), e.g., from individual sources, have exhibited the unique characteristics of self-renewal capacity and multipotential differentiation. Nevertheless, each type has pros and cons as an available source for regenerative cell-based therapies. In particular, there are several serious concerns including ethical issues, tumor formation, and genetic instability, thus undermining their therapeutic application [8,9,10]. These are mainly attributable to the generation of iPSCs with several exogenous genetic manipulations, destroying the embryo in harvesting ESCs and generating teratoma arising from intermediate pluripotent cell state after transplantation [10, 11]. Additionally, current protocols for differentiating these types of stem cells into lineage-specific neurons such as DA neurons usually have poor conversion efficiency and are highly involved in terms of time consumption, technical complexity, and induction materials [12,13,14]. Therefore, it is imperative to seek an alternative stem cell source that can generate DA neurons while being free of evident disadvantages such as ethical issues and tumor formation. Meanwhile, it is of great importance to establish a highly efficient and cost-effective differentiation strategy to generate functional DA neurons from stem cells.
Spermatogonial stem cells (SSCs) are a subpopulation of type A spermatogonia [15, 16]. SSCs were previously regarded as unipotent stem cells, differentiating into sperm only. With further studies on SSCs, however, the previously prevailing orthodoxy has been challenged. To date, a growing number of studies have demonstrated that SSCs from both mouse and human testes can dedifferentiate into ES-like cells that are capable of giving rise to various cell lineages of all three germ layers [17,18,19,20,21,22,23], implying that SSCs may be an alternative cell source for regenerative medicine. In addition, SSCs have several merits over other types of stem cells, including lack of ethical issues, lower tumorigenesis, and no immunogenicity [24]. More notably, SSCs can transdifferentiate into uterine, prostatic, and skin epithelium cells in vivo after transplantation into these tissues, mainly due to the strong sensitivity of SSC to living microenvironments [21]. Thus, SSCs could serve as another potential source for replacing damaged or degenerated cells in various neurodegenerative diseases. However, direct conversion of hSSCs to functional DA neurons without forced expression of transcription factors or reprogramming to a pluripotent state has not been achieved in vitro.
Here, we describe a straightforward two-step induction strategy for differentiating hSSCs into DA neurons based on unique characteristics of SSCs. Using this protocol, we achieved the direct conversion of hSSCs into DA neurons that recapitulated the morphological, key biochemical, and functional features of primary midbrain DA neurons. Notably, the acquisition of hSSC-derived DA neurons using OECCM and a set of defined cell-extrinsic factors as well as several small molecules could be much safer than other options. On the other hand, the newly established differentiation protocol obviates a complicated manipulation procedure and reduces the overall cost of the differentiation process. The present groundbreaking study may open the possibility of generating human DA neurons from hSSCs for PD therapy.
Materials and methods
Preparation of primary hSSCs
Testicular tissues from obstructive azoospermic (OA) patients at the age of 25–45 years who had normal spermatogenesis and needed microdissection for testicular sperm extraction were fully washed in DMEM/F12 (Gibco) with antibiotics (Gibco). Importantly, all experiments were performed in accordance with the relevant guidelines and regulations of the Institutional Ethical Review Committee of Hong Hui Hospital affiliated to ** in the cylinder, while the reduced activity was inhibited by engrafted hSSCs. Statistical analysis indicated that there was a significant difference between the mock and hSSC transplantation groups, and no significant difference was found between the normal and hSSCs transplantation groups (Fig. 8a). Moreover, alterations in spontaneous activity to remove the label were reliably observed after hSSC transplantation. Strikingly, MPTP-treated animals engrafted with hSSCs showed significant improvement in adhesive removal performance compared with mock animals, displaying a significantly shortened time to remove the label using their forepaws. Additionally, there was a statistical difference between engrafted hSSCs of MPTP-treated and normal animals (Fig. 8b), indicating the therapeutic potential of hSSCs. Consistent with the spontaneous activity test, the gait analysis indicated that MPTP-treated mice showed a progressive decline in the walking distance during the unit time (2 min), whereas engraftment of hSSCs remarkably impeded this declining trend. Nevertheless, no significant difference was found at any of the time points posttransplantation (Fig. 8c). Likewise, the walking velocity of MPTP-treated animal was also increased by hSSC engraftment and showed an increasing tendency with prolongation of transplantation time. Strikingly, the significant increase occurred mainly beyond 4 weeks posttransplantation; however, no significant difference was observed between the hSSC transplantation and normal groups although there was an increasing trend in the walking velocity in hSSC transplantation group (Fig. 8d). In addition, analysis of challenging beam assays indicated that MPTP-treated mice showed a significant increase in errors per step compared to normal mice, while the parameter regarding errors per step was remarkably decreased by hSSC engraftment at 6 weeks posttransplantation (Fig. 8e). When animals were tested on a beam that tapered from wide to narrow, similar results were obtained (Fig. 8f), demonstrating their propensity to make errors was reduced. Additionally, the number of amphetamine-induced rotations following MPTP injection in animals transplanted pre-induced hSSCs decreased (at 1, 2, and 3 weeks posttransplantation: 899 ± 185 turns/90 min, 756 ± 215 turns/90 min, and 560 ± 235 turns/90 min, respectively) compared to control group (1033 ± 243 turns/90 min). Notably, the number of amphetamine-induced rotations significantly decreased in the animals transplanted pre-induced hSSCs for 3 weeks compared to that in control group (p < 0.05) (Fig. 8g).
Behavioral deficit improvement after hSSC transplantation. a Hindlimb step** in the cylinder in normal (n = 10), hSSC-transplanted (n = 8), and mock (saline-injected, n = 6) mice at 7 weeks postoperation. b Contact time in normal (n = 10) and hSSC-transplanted (n = 8), and mock (saline-injected, n = 5) mice at 7 weeks postoperation. “*” and “#” represent p < 0.05 compared to mock and normal, respectively. c, d Distance traveled in unit time and movement velocity in normal (n = 10), hSSCs-transplanted (n = 8), and mock (saline-injected, n = 6) mice at postoperation. e Errors per step in the challenging beam of the abovementioned mice at 7 weeks postoperation. f Mean errors at different beam widths. g The number of amphetamine-induced rotation in hSSCs groups, compared to that of the control group (mock). “*” and “**” represent p < 0.05 and p < 0.01, respectively, compared to mock. Each animal received at least five trials. “#” and “##” represent p < 0.05 and p < 0.01, respectively, compared to normal
Discussion
The progressive degeneration and further loss of DA neurons in the midbrain is the main pathological process of PD, generally causing motor and sensory dysfunction [41, 42]. Regardless of the temporary amelioration of symptom by current therapeutic approaches (drug, gene, surgery and deep-brain stimulation, etc.), no effective treatments hitherto can cure the disease. On the basis of the properties of PD, cell replenishment therapy has been proposed as a promising treatment strategy for PD. Despite the therapeutic potential of a variety of stem cells including NSCs, their availability is extremely limited, mainly due to their individual shortcomings, including low accessibility and expansion and ethical and immune concerns [39]. Although ESCs, iPSCs, and MSCs hold great potential in regenerative medicine, their clinical application could not circumvent the risk for undesired genotoxicity, mutagenesis, and tumorigenesis besides differentiation uncertainty and ethical controversies [9,10,11, 43]. For this reason, finding a desirable cell-based therapy for PD is essential. Recently, advances in stem cell reprogramming studies have revealed that SSCs can transdifferentiate into neural cells, cardiovascular cells, and prostatic, uterine, and skin epithelial cells in addition to sperm lineage cells [17, 19, 22, 24, 44, 45]. This unique differentiation property is mainly attributed to both SSC-intrinsic and SSC-extrinsic factors [21]. In addition, several previous reports together with our recent studies demonstrated that SSCs have substantial advantages over other types of stem cells, such as rapid expansion, strong multipotential, and high amenability to the extrinsic environment [24, 46, 47]. More importantly, in comparison to several types of primary stem cells, SSCs have several attractive features, including no ethical concerns, lower tumorigenicity and host immune response, and wide availability [48], thus avoiding current obstacles and risks of autologous or allogenetic transplantation of SSCs. Therefore, we speculate that SSCs are likely to be the best prospective candidates for clinical applications. Nonetheless, there is not a highly successful and efficient method of reprogramming hSSCs to DA neurons.
In the present study, we developed an efficient and straightforward protocol for the conversion of hSSCs into functional DA neurons. The converted SSCs exhibited typical neuronal morphology accompanied by biochemical phenotypes of midbrain DA neurons, as well as the ability to secrete dopamine and initiate neuron-specific electrophysiology activity and calcium imaging. Following the in vitro study, the therapeutic potential of the converted cells from hSSCs was specifically supported in our in vivo experiment by behavioral improvement in the MPTP-lesioned mice after striatal cell transplantation. Safety, efficacy, and practicability will be of critical importance in develo** a novel strategy for PD clinical therapy. Based on this, we tried to avoid the deficits and undesired side effects in the conventional protocol and further optimize the tedious procedure by relying on the high amenability of SSCs to the extrinsic environment. To develop a highly efficient approach for DA neuron conversion in hSSCs, apart from the delivery of traditional induction factors, such as GDNF, RA, and forskolin, OECCM and several defined components (SHH, TGFβ3, FGF8α, VPA, and SB) that effectively promote reprogramming events, including epigenetic dynamics, neurogenesis, survival, neuron subtype specification, and maturation, were supplemented. This transdifferentiation protocol for converting hSSC to DA neurons circumvents conventional drawbacks: inefficiency, tedious multistep processes, and the high risk of exogenous gene delivery. In summary, we sought to develop the hSSC induction system for future clinical consideration.
In this study, we demonstrated that this induction system is particularly amenable to giving rise to a large number of functional DA neurons as demonstrated by significant dopaminergic marker expression and functional properties after 2 and 3 weeks of in vitro induction. To gain novel insights into the molecular mechanisms underlying the neural conversion of hSSCs, we illustrate the principles of establishing this induction system, relying on the roles of each component in the conversion. Herein, OECCM was used as a critical basic component from the OEC culture supernatant. Numerous studies have demonstrated that OECs can secrete a variety of neurotrophic growth factors and cell adhesion molecules, some of which play pivotal roles in directing neural differentiation, survival, maturation, and migration and further maintenance of differentiated neuronal phenotypes [49,50,51,52,53,54]. Similar to the claim, our recent study also revealed that OECCM significantly improved adipose-derived stem cell and bone mesenchymal stem cell transdifferentiation efficiency [55, 56]; therefore, OECCM includes the induction system. The use of a defined set of factors, including SHH, GDNF, FGF8α, and TGFβ3, plays pivotal roles in neurogenesis and differentiation. In particular, some factors (e.g., SHH and FGF8α) are requisite for subtype specification of differentiating neurons during the embryonic brain development stage [57,58,59,60,61], since FoxA2 and Lmxla/b, two important effectors in downstream of the SHH signaling pathway, have been shown to effectively convert differentiating neural cells into DA neurons and maintain the phenotype specification [62, 63]. The presence of GDNF in the induction system mainly attributes its biofunctions to midbrain dopaminergic neurons [64, 65], such as the promotion of proliferation and specification, neurite growth, synaptic and electrophysiological maturation, soma expansion and expression of phenotype-specific proteins as well as regulating downstream effector genes. Of critical importance is our application of three components (e.g., TGFβ3, VPA and SB) required for cell lineage transdifferentiation [66, 67]. Other combined factors may also play synergistical roles in hSSC reprogramming. As a result, epigenetic switching caused by a complex interaction among these distinct molecules eventually achieves the conversion of hSSCs into DA neurons.
Although it is unclear how the minimal set of defined factors induce hSSCs to transdifferentiate into DA neurons in a short time frame, our present results have shown that our approach is efficient for generating a high number of functional DA neurons both in vitro and in vivo. To further elaborate the conversion, we assayed global transcriptome profiles and activation of several crucial proneurogenic factors before and after hSSC transdifferentiation in addition to conventional morphological traits and phenotypic characteristics. More importantly, it is imperative to determine whether the converted cells function as genuine DA neurons (e.g., synapse formation, dopamine release, neuron-specific calcium imaging and electrophysiology, and rescuing deficits after grafted in an animal model of PD). As expected, these converted hSSCs acquire cellular characteristics that assemble those of genuine DA neurons without undergoing an ESC process. Strikingly, the transdifferentiation of hSSCs toward DA neurons exhibits high efficiency due to dramatic epigenetic alterations revealed by a gain of neural cell attributes and loss of SSC fate. In particular, there is a high similarity in epigenetic genes (accounting for 88.9%) and activation of several key determinants (e.g., EN-1, Pitx3, Foxa2, Lmx1a/b, and OTX2) required for the development and specification of DA neurons [62]. The results indicate that the induction process appears to mimic the dopaminergic neurogenesis that occurs during embryonic development, albeit with limited precision. In addition, we traced the fate of engrafted hSSCs up to 4 weeks posttransplantation and found that the engrafted hSSCs could differentiate into DA neuronal lineages without forming tumors. This is likely to due to direct in vivo transdifferentiation of SSCs without ESC or intermediate precursor process, which have been strengthened by double staining for nestin and cyclinD1 in the grafts. Inspiringly, the grafted cells exhibited extensive arborization and irregular somata with robust neurite and remarkably enhanced behavioral improvement in MPTP-lesioned mice as compared to controls. Our results further revealed excellent safety and efficacy in a rodent model of PD using SSC-derived DA neurons, which may be due to direct induction without the introduction of ectopic genes. This finding has also been supported by our previous reports [20]. Thus, the present study may provide an implication for the application of SSC-based therapeutic covering a vast array of regenerative medicine disciplines.
Conclusions
In summary, this results presented here showed that we successfully induced the differentiation of hSSCs to DA neurons using OECCM combined with a set of defined factors. The differentiated cells exhibited morphology and functionality similar to genuine DA neurons, such as spontaneous action potentials, DA release, and rescuing behavioral deficits of PD animals. Of unusual significance, the direct conversion of hSSCs to DA neurons by means of several active molecules without the instruction of exogenous genes is thus relatively safe and circumvents numerous concerns in future clinical applications. Overall, this innovative approach provides an extremely attractive solution to significant deficits in PD cell-replacement therapy. Also, this approach may serve as a general strategy for the generation of many distinct neuronal subtypes.
Availability of data and materials
The datasets presented in these studies are available from the corresponding author upon request.
Abbreviations
- ACSF:
-
Artificial cerebrospinal fluid
- bFGF:
-
Basic fibroblast growth factor
- BIC:
-
Bicuculline
- CD133:
-
Prominin-1
- C-myc:
-
Proto-oncogene
- CNP:
-
2′,3′-Cyclic nucleotide 3′phosphodiesterase
- DA:
-
Dopaminergic
- DAPI:
-
4′-6-Diamidino-2-phenylindole
- DAT:
-
Dopamine transporter
- DAZL:
-
Deleted in azoospermia-like protein
- EGF:
-
Epidermal growth factor
- EN-1:
-
Engrailed-1
- ESCs:
-
Embryonic cells
- FBS:
-
Fetal bovine serum
- FoxA2:
-
Forkhead box protein A2
- GDNF:
-
Glial cell-derived neurotrophic factor
- GFAP:
-
Glial fibrillary acidic protein
- GFRA1:
-
GDNF family receptor alpha 1
- GPR125:
-
G protein-coupled receptor 125
- HNu:
-
Human nuclear protein
- hSSCs:
-
Human spermatogonial stem cells
- iPSCs:
-
Induced pluripotent stem cells
- LIF:
-
Leukemia inhibitory factor
- Lmx1a:
-
LIM homeobox transcription factor 1 alpha
- Lmx1b:
-
LIM homeobox transcription factor 1 alpha
- MSCs:
-
Mesenchymal stem cells
- NSCs:
-
Neural stem cells
- Nurr1:
-
Orphan member of the nuclear receptor superfamily 1
- OEC:
-
Olfactory ensheathing cell
- OECCM:
-
Olfactory ensheathing cell conditioned culture medium
- OTX2:
-
Orthodenticle homeobox 2
- Pax-6:
-
Paired box protein 6
- PCNA:
-
Proliferating cell nuclear antigen 1
- PD:
-
Parkinson’s disease
- PitX3:
-
Pituitary homeobox 3
- PLZF:
-
Promyelocytic leukemia zinc finger
- SHH:
-
Sonic hedgehog
- SSEA-1:
-
Stage-specific embryonic antigen 1
- SYN:
-
Synapsin
- TEA:
-
Tetraethylammonium chloride
- TGF8α:
-
Transforming growth factor 8 alpha
- TGFβ3:
-
Transforming growth factor beta 3
- TH:
-
Tyrosine hydroxylase
- TTX:
-
Tetrodotoxin
- Tuj-1:
-
Beta3-tubulin
- UCHL1:
-
Ubiquitin C-terminal hydrolase L1
- VPA:
-
Valproate sodium
References
Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912.
Chinta SJ, Kumar MJ, Hsu M, Rajagopalan S, Kaur D, Rane A, Nicholls DG, Choi J, Andersen JK. Inducible alterations of glutathione levels in adult dopaminergic midbrain neurons result in nigrostriatal degeneration. J Neurosci. 2007;27:13997–4006.
Muhammed K, Manohar S, Husain M. Mechanisms underlying apathy in Parkinson’s disease. Lancet. 2015;385:S71.
Miyasaki JM, Martin W, Suchowersky O, Weiner WJ, Lang AE. Practice parameter: initiation of treatment for Parkinson’s disease: an evidence-based review: report of the quality standards Subcommittee of the American Academy of neurology. Neurol. 2002;58:11–7.
Goetz CG, Poewe W, Rascol O, Sampaio C. Evidence-based medical review update: pharmacological and surgical treatments of Parkinson’s disease: 2001 to 2004. Mov Disord. 2005;20:523–39.
Ossig C, Reichmann H. Treatment of Parkinson’s disease in the advanced stage. J Neural Transm (Vienna). 2013;120:523–9.
Fernandez HH, Chen JJ. Monoamine oxidase-B inhibition in the treatment of Parkinson’s disease. Pharmacotherapy. 2007;27(12 Pt 2):174S–85S.
Lee AS, Tang C, Rao MS, Weissman IL, Wu JC. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med. 2013;19:998–1004.
Knoepfler PS. Deconstructing stem cell tumorigenicity: a roadmap to safe regenerative medicine. Stem Cells. 2009;27:1050–6.
Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, Weinberg RA. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507.
Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, Armstrong L, Djonov V, Lako M, Stojkovic M. Ethical and safety issues of stem cell-based therapy. Int J Med Sci. 2018;15:36–45.
Li Y, Welm B, Podsypanina K, Huang S, Chamorro M, Zhang X, Rowlands T, Egeblad M, Cowin P, Werb Z, et al. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci U S A. 2003;100:15853–8.
Espuny-Camacho I, Michelsen KA, Gall D, Linaro D, Hasche A, Bonnefont J, Nakagawa M, Koyanagi M, Tanabe K, Ohnuki M, et al. Pyramidal neurons derived from human pluripotent stem cells integrate efficiently into mouse brain circuits in vivo. Neuron. 2013;77:440–56.
Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743–5.
Oatley JM, Avarbock MR, Telaranta AI, Fearon DT, Brinster RL. Identifying genes important for spermatogonial stem cell self-renewal and survival. Proc Natl Acad Sci U S A. 2006;103:9524–9.
Kanatsu-Shinohara M, Shinohara T. Spermatogonial stem cell self-renewal and development. Annu Rev Cell Dev Biol. 2013;29:163–87.
Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, Nolte J, Wolf F, Li M, Engel W, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–203.
Nazm Bojnordi M, Movahedin M, Tiraihi T, Javan M, Ghasemi Hamidabadi H. Oligoprogenitor cells derived from spermatogonia stem cells improve remyelination in demyelination model. Mol Biotechnol. 2014;56:387–93.
Glaser T, Opitz T, Kischlat T, Konang R, Sasse P, Fleischmann BK, Engel W, Nayernia K, Brüstle O. Adult germ line stem cells as a source of functional neurons and glia. Stem Cells. 2008;26:2434–43.
Kim BJ, Lee YA, Kim KJ, Kim YH, Jung MS, Ha SJ, Kang HG, Jung SE, Kim BG, Choi YR, et al. Effects of paracrine factors on CD24 expression and neural differentiation of male germline stem cells. Int J Mol Med. 2015;36(1):255–62.
Simon L, Ekman GC, Kostereva N, Zhang Z, Hess RA, Hofmann MC, Cooke PS. Direct transdifferentiation of stem/progenitor spermatogonia into reproductive and nonreproductive tissues of all germ layers. Stem Cells. 2009;27:1666–75.
Yang H, Liu C, Chen B, An J, Zhang R, Zhang Q, Zhao J, He BR, Hao DJ. Efficient generation of functionally active spinal cord neurons from spermatogonial stem cells. Mol Neurobiol. 2017;54(1):788–803.
Lee HJ, Choi NY, Lee SW, Ko K, Hwang TS, Han DW, Lim J, Schöler HR, Ko K. Epigenetic alteration of imprinted genes during neural differentiation of germline-derived pluripotent stem cells. Epigenetics. 2016;11:177–83.
Yang H, Liu Y, Hai Y, Guo Y, Yang S, Li Z, He Z. Efficient conversion of spermatogonial stem cells to phenotypic and functional dopaminergic neurons via the PI3K/Akt and P21/Smurf2/Nolz1 pathway. Mol Neurobiol. 2015;52:1654–69.
He BR, Lu F, Zhang LL, Hao DJ, Yang H. An alternative long-term culture system for highly-pure mouse spermatogonial stem cells. J Cell Physiol. 2015;230:1365–75.
He BR, **e ST, Wu MM, Hao DJ, Yang H. Phagocytic removal of neuronal debris by olfactory ensheathing cells enhances neuronal survival and neurite outgrowth via p38MAPK activity. Mol Neurobiol. 2014;49:1501–12.
Yang H, Cheng XP, Li JW, Yao Q, Ju G. De-differentiation response of cultured astrocytes toinjury induced by scratch or conditioned culture medium of scratch-insulted astrocytes. Cell Mol Neurobiol. 2009;29:455–73.
Yang H, Cheng XP, Yao Q, Li JW, Ju G. The promotive effects of the thymosin-β on neuronal survival and neurite outgrowth by upregulating L1 expression. Neurochem Res. 2008;33:2269–80.
Thier M, Wörsdörfer P, Lakes YB, Gorris R, Herms S, Opitz T, Seiferling D, Quandel T, Hoffmann P, Nöthen MM, et al. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell. 2012;10:473–9.
Liu H, Wang ZL, Tian LQ, Qin QH, Wu XB, Yan WY, Zeng ZJ. Transcriptome differences in the hypopharyngeal gland between Western Honeybees (Apis mellifera) and Eastern Honeybees (Apis cerana). BMC Genomics. 2014;15:744.
Guo L, Karoubi G, Duchesneau P, Shutova MV, Sung HK, Tonge P, Bear C, Rogers I, Nagy A, Waddell TK. Generation of induced progenitor-like cells from mature epithelial cells using interrupted reprogramming. Stem Cell Reports. 2017;9:1780–95.
Son EY, Ichida JK, Wainger BJ, Toma JS, Rafuse VF, Woolf CJ, Eggan K. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell. 2011;9:205–18.
Xu HM, Wang YH, He ZP, Yang H, Gao WQ. Direct conversion of mouse fibroblasts to GABAergic neurons with combined medium without the introduction of transcription factors or miRNAs. Cell Cycle. 2015;14:2451–60.
Tropel P, Platet N, Platel JC, Noël D, Albrieux M, Benabid AL. Functional neural differentiation of bone marrow derived mesenchymal stem cells. Stem Cells. 2006;24:2868–76.
Chen PC, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, Kan YW, Johnson JA. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson's disease: critical role for the astrocyte. Proc Natl Acad Sci U S A. 2009;106:2933–8.
da Conceição FS, Ngo-Abdalla S, Houzel JC, Rehen SK. Murine model for Parkinson’s disease: from 6-OH dopamine lesion to behavioral test. J Vis Exp. 2010;35:1376.
Glajch KE, Fleming SM, Surmeier DJ, Osten P. Sensorimotor assessment of the unilateral 6-hydroxydopamine mouse model of Parkinson’s disease. Behav Brain Res. 2012;230:309–16.
Fleming SM, Ekhator OR, Ghisays V. Assessment of sensorimotor function in mouse models of Parkinson’s disease. J Vis Exp. 2013;76: e50303. https://doi.org/10.3791/50303.
Corti S, Nizzardo M, Simone C, Falcone M, Donadoni C, Salani S, Rizzo F, Nardini M, Riboldi G, Magri F, et al. Direct reprogramming of human astrocytes into neural stem cells and neurons. Exp Cell Res. 2012;318:1528–41.
Smith GA, Dunnett SB, Lane EL. Amphetamine-induced rotation in the transplanted hemi-parkinsonian rat--response to pharmacological modulation. Behav Brain Res. 2012;232:411–5.
Rodriguez-Oroz MC, Jahanshahi M, Krack P, Litvan I, Macias R, Bezard E, Obeso JA. Initial clinical manifestations of Parkinson's disease: features and pathophysiological mechanisms. Lancet Neurol. 2009;8:1128–39.40.
Dexter DT, Jenner P. Parkinson disease: from pathology to molecular disease mechanisms. Free Radic Biol Med. 2013;62:132–44.
Gracia CR, Woodruff T. Use of very small embryonic-like stem cells to avoid legal, ethical, and safety issues associated with oncofertility-reply. JAMA Oncol. 2016;2:689–90.
Guan K, Wagner S, Unsöld B, Maier LS, Kaiser D, Hemmerlein B, Nayernia K, Engel W, Hasenfuss G. Generation of functional cardiomyocytes from adult mouse spermatogonial stem cells. Circ Res. 2007;100:1615–25.
Streckfuss-Bömeke K, Vlasov A, Hülsmann S, Yin D, Nayernia K, Engel W, Hasenfuss G, Guan K. Generation of functional neurons and glia from multipotent adult mouse germ-line stem cells. Stem Cell Res. 2009;2:139–54.
Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 2004;101:16489–94.
Kubota H, Wu X, Goodyear SM, Avarbock MR, Brinster RL. Glial cell line-derived neurotrophic factor and endothelial cells promote self-renewal of rabbit germ cells with spermatogonial stem cell properties. FASEB J. 2011;25:2604–14.
Zhang Z, Gong Y, Guo Y, Hai Y, Yang H, Yang S, Liu Y, Ma M, Liu L, Li Z, et al. Direct transdifferentiation of spermatogonial stem cells to morphological, phenotypic and functional hepatocyte-like cells via the ERK1/2 and Smad2/3 signaling pathways and the inactivation of cyclin A, cyclin B and cyclin E. Cell Commun Signal. 2013;11:67.
Plant GW, Harvey AR, Leaver SG, Lee SV. Olfactory ensheathing glia: repairing injury to the mammalian visual system. Exp Neurol. 2011;229:99–108.
Kocsis JD, Lankford KL, Sasaki M, Radtke C. Unique in vivo properties of olfactory ensheathing cells that may contribute to neural repair and protection following spinal cord injury. Neurosci Lett. 2009;456:137–42.
Cao L, Su Z, Zhou Q, Lv B, Liu X, Jiao L, Li Z, Zhu Y, Huang Z, Huang A, et al. Glial cell line-derived neurotrophic factor promotes olfactory ensheathing cells migration. Glia. 2006;54:536–44.
Runyan SA, Phelps PE. Mouse olfactory ensheathing glia enhance axon outgrowth on a myelin substrate in vitro. Exp Neurol. 2009;216:95–104.
Shukla S, Chaturvedi RK, Seth K, Roy NS, Agrawal AK. Enhanced survival and function of neural stem cells-derived dopaminergic neurons under influence of olfactory ensheathing cells in parkinsonian rats. J Neurochem. 2009;109:436–51.
Jiao Y, Novozhilova E, Karlén A, Muhr J, Olivius P. Olfactory ensheathing cells promote neurite outgrowth from co-cultured brain stem slice. Exp Neurol. 2011;229:65–71.
**e ST, Lu F, Han JT, Tao K, Wang HT, Simental A, Hu D, Yang H. Efficient generation of functional Schwann cells from adipose-derived stem cells in defined conditions. Cell Cycle. 2017;16:841–51.
**e ST, Lu F, Zhang XJ, Shen Q, He ZP, Gao WQ, Yang H. Retinoic acid and human olfactory ensheathing cells cooperate to promote neural induction from human bone marrow stromal stem cells. NeuroMolecular Med. 2013;15:252–64.
Martí E, Bovolenta P. Sonic hedgehog in CNS development: one signal, multiple outputs. Trends Neurosci. 2002;25:89–96.
Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol. 2000;18:675–9.
Briscoe J, Sussel L, Serup P, Hartigan-O'Connor D, Jessell TM, Rubenstein JL, Ericson J. Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature. 1999;398:622–7.
Ishibashi M, McMahon AP. A sonic hedgehog dependent signaling relay regulates growth of diencephalic and mesencephalic primordia in the early mouse embryo. Development. 2002;129:4807–19.
Holzschuh J, Hauptmann G, Driever W. Genetic analysis of the roles of Hh, FGF8, and nodal signaling during catecholaminergic system development in the zebrafish brain. J Neurosci. 2003;23:5507–19.
Hong S, Chung S, Leung K, Hwang I, Moon J, Kim KS. Functional roles of Nurr1, Pitx3, and Lmx1a in neurogenesis and phenotype specification of dopamine neurons during in vitro differentiation of embryonic stem cells. Stem Cells Dev. 2014;23:477–87.
Zhou X, Pace J, Filichia E, Lv T, Davis B, Hoffer B, Selman W, Luo Y. Effect of the sonic hedgehog receptor smoothened on the survival and function of dopaminergic neurons. Exp Neurol. 2016;283:235–45.
Drinkut A, Tillack K, Meka DP, Schulz JB, Kügler S, Kramer ER. Ret is essential to mediate GDNF’s neuroprotective and neuroregenerative effect in a Parkinson disease mouse model. Cell Death Dis. 2016;7:e2359.
Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–2.
Roussa E, Oehlke O, Rahhal B, Heermann S, Heidrich S, Wiehle M, et al. Transforming growth factor beta cooperates with persephin for dopaminergic phenotype induction. Stem Cells. 2008;26:1683–94.
Takayama Y, Wakabayashi T, Kushige H, Saito Y, Shibuya Y, Shibata S, Akamatsu W, Okano H, Kida YS. Brief exposure to small molecules allows induction of mouse embryonic fibroblasts into neural crest-like precursors. FEBS Lett. 2017;591:590–602.
Acknowledgements
We would like to acknowledge Huiming Xu, Yonghui Wang, and Minghui Niu for the excellent technical assistance. We would like to thank Dr. Sebastian Schmull for critical reading of the manuscript.
Funding
This work was supported by the Natural Science Foundation of China (grant nos. 81371411, 81571208, 81830077, and 81772357), the Natural Science Foundation of Shaanxi province (2017JM8012), Shanghai Jiaotong University Med-X Fund (No.YG2014MS45), and a key fund of Honghui Hospital (YJ2017001).
Author information
Authors and Affiliations
Contributions
HY and DHa conceived the study and designed the work. HY,ChL, DHa, DHu, and HF performed the experiments. CuL, ChL, BC, LZ, QZ, and JA collected and analyzed the data. DHa, CuL, BC, QZ, JZ, and HY performed data analysis and interpretation. DHa and HY wrote the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Human testicular tissues were obtained from clinically obstructive azoospermic (OA) patients who had normal spermatogenesis and all corpse subjects for autopsy and histopathological assessment in accordance with local and Nation Ethics committee approved. All animal procedures were performed in agreement with the National Institutes of Health guidelines and were approved by the Institutional Ethical Review Committee of Hong Hui Hospital affiliated by **’an Jiaotong University). Written informed consent was obtained from all participants involved in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Yang, H., Hao, D., Liu, C. et al. Generation of functional dopaminergic neurons from human spermatogonial stem cells to rescue parkinsonian phenotypes. Stem Cell Res Ther 10, 195 (2019). https://doi.org/10.1186/s13287-019-1294-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-019-1294-x