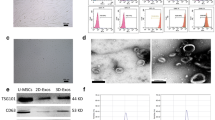
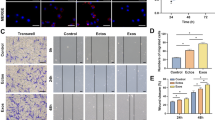
Abstract
Background
Develo** cartilage constructed with the appropriate matrix composition and persistent chondrogenesis remains an enduring challenge in cartilage defects. Cartilage progenitor cell (CPC)-based tissue engineering has attracted recent attention because of its strong chondrogenic differentiation capacity. However, due to the lack of a suitable chondrogenic niche, the clinical application of CPC-regenerated cartilage in the subcutaneous environment remains a challenge. In this study, exosomes derived from chondrocytes (CC-Exos) were used to provide the CPC constructs with a cartilage signal in subcutaneous environments for efficient ectopic cartilage regeneration.
Methods
Rabbit CPC-alginate constructs were prepared and implanted subcutaneously in nude mice. CC-Exos were injected into the constructs at the same dose (30 μg exosomes per 100 μL injection) after surgery and thereafter weekly for a period of 12 weeks. Exosomes derived from bone mesenchymal stem cells (BMSC-Exos) were used as the positive control. The mice in the negative control were administered with the same volume of PBS. At 4 and 12 weeks after implantation, the potential of CC-Exos and BMSC-Exos to promote chondrogenesis and stability of cartilage tissue in a subcutaneous environment were analyzed by histology, immunostaining, and protein analysis. The influences of BMSC-Exos and CC-Exos on chondrogenesis and angiogenic characteristics in vitro were assessed via coculturing with CPCs and human umbilical vein endothelial cells.
Results
The CC-Exos injection increased collagen deposition and minimized vascular ingrowth in engineered constructs, which efficiently and reproducibly developed into cartilage. The generated cartilage was phenotypically stable with minimal hypertrophy and vessel ingrowth up to 12 weeks, while the cartilage formed with BMSC-Exos was characterized by hypertrophic differentiation accompanied by vascular ingrowth. In vitro experiments indicated that CC-Exos stimulated CPCs proliferation and increased expression of chondrogenesis markers while inhibiting angiogenesis.
Conclusions
These findings suggest that the novel CC-Exos provides the preferable niche in directing stable ectopic chondrogenesis of CPCs. The use of CC-Exos may represent an off-the-shelf and cell-free therapeutic approach for promoting cartilage regeneration in the subcutaneous environment.
Similar content being viewed by others
Background
The structural and functional repair of sizeable subcutaneous cartilage defects remains a challenge in plastic and reconstructive surgery [1, 50,51], which was fraught with a couple of shortcomings [5, 52, 53]. Tissue-engineered cartilage grafts have emerged as a promising alternative to overcome these problems and satisfy the ever-increasing clinical need [54,55,56]. Currently, exosomes have been identified as the principal agent in mediating the therapeutic efficacy of the cell-based regenerative medicine approach [20, 21, 57, 58], and BMSC-Exos have been reported for promoting in situ cartilage defect repair [21, 26]. However, the therapeutic outcome for subcutaneous cartilage defect repair is still limited because of the lack of a suitable pro-chondrogenic environment [17, 40, 59]. Meanwhile, previous studies have also shown that chondrocytes could steer the chondrogenesis of stem cells in vitro and in vivo through paracrine effects [1, 7, 60]. In the present study, using a CPC-based cartilage tissue engineering approach, the potential of CC-Exos in promoting ectopia chondrogenesis and stabilizing cartilage regeneration in a subcutaneous environment was further investigated.
The current study demonstrated that CPC constructs supplied with CC-Exos could form homogeneous cartilage-like tissue with minimal hypertrophy in a subcutaneous environment, with no help from any chondrogenic factors. Furthermore, a series of in vitro experiments further confirmed that CC-Exos significantly promoted chondrogenesis-related factors at the mRNA and protein levels in CPCs, such as SOX-9 and COL II. Importantly, angiogenesis was inhibited by CC-Exos, which is known to be detrimental to cartilage regeneration leading to hypertrophic differentiation and subsequent calcification [44]. The observed contributions of CC-Exos to cartilage regeneration in vivo corroborate the in vitro findings and further support that CC-Exos alone could provide a preferable chondrogenic environment and help maintain the stability of cartilage tissue. Compared with BMSC-Exos where samples show more hypertrophic cartilage, the cartilage regeneration results achieved with the use of CC-Exos are significantly more favorable. Hence, the null hypothesis that there is no difference between CC-Exos and BMSC-Exos in cartilage regeneration results must be rejected.
To date, tissue engineering has offered promising solutions for clinical issues involving congenital and acquired cartilage defects [7, 61, 62]. However, the cartilage formation in subcutaneous environments is limited due to the lack of a proper chondrogenic niche [11, 12, 59]. Imitating the chondrogenic niche is a well-accepted approach to promote the ectopic chondrogenesis of progenitor cells [16, 18]. Exosomes have been studied extensively for their potential in participating in the maintenance of normal physiology via delivering various types of bioactive microRNAs, nucleic acids, proteins, and unique gene products [22, 63]. Recent studies have shown that chondrocytes and chondrocyte-related factors play key regulatory roles in the maintenance of the cartilage microenvironment and the ultimate cartilage phenotype of implanted stem cells [13,14,15]. In the present study, we further demonstrate that CC-Exos modulates CPC migration, proliferation, and cartilage matrix synthesis. Expression of SOX-9 and COL II by CPCs is upregulated in the presence of CC-Exos, which promotes chondrogenesis. This may be attributed to the TGF-β/SMAD signaling pathway, which is reported to play an essential role in chondrocyte differentiation and matrix maturation [41, 45, 64]. More investigations are needed to acquire the whole picture of the pathway involved in CC-Exos-induced chondrogenesis.
Additionally, reproducibly generating stable cartilage remains an unsolved challenge. Avoiding vessel ingrowth and hypertrophy is a critical factor in building stable cartilage [65, 66]. In the present study, compared to the positive control groups (BMSC-Exos), CC-Exos could maintain a stabilized phenotype of constructed cartilage at least within the investigated time frame, as evidenced by the presence of significantly less COL X-positive staining and minimal protein expression of COL X, IHH, and MMP 13 secreted by hypertrophic chondrocytes at 12 weeks. In addition, less CD31-positive microvessels are observed in the neo-cartilage of the CC-Exos group. However, after the addition of BMSC-Exos, expression of SDF-1 and VEGF is upregulated, which promotes cell homing and angiogenesis. This is beneficial for cartilage engineering during the early stage of implantation [40], which may account for the better neo-cartilage formation in the BMSC-Exos, as reported [21, 26]. However, it also has a disadvantage as evidenced by promoting associated ectopic cartilage hypertrophy. Recent studies have also shown that vascular invasion is one of the major mechanisms involved in hypertrophic cartilage differentiation [5, 44]. In vitro results also revealed that HUVEC migration and tube formation are reduced by CC-Exos when compared with BMSC-Exos. These results collaborate with data collected from an in vivo experiment, which shows CC-Exos have the ability to decrease angiogenesis in subcutaneous cartilage repair. Because CC-Exos can promote CPC migration, proliferation, and matrix synthesis in vitro, a more favorable prognosis is anticipated for long-term cartilage regeneration.
A novel method of imitating the chondrogenic niche is explored in the present work via the use of CC-Exos. After local injection of CC-Exos, the CPCs are rapidly directed to form neo-cartilage, when stimulated by chondroinductive mediators. Importantly, the engineered cartilage here can maintain the stabilized phenotype in non-chondrogenic niches, which is probably related to antiangiogenic factors secreted by CC-Exos that prevent neovascularization and hypertrophy. Because strategies that provide the conventional cartilage environment often require cell-based therapy [54, 55], the use of CC-Exos is advantageous from the perspectives of off-the-shelf and cell-free regenerative medicine approach for cartilage repair, and the ease of minimally invasive injection of CC-Exos concentrate.
Despite these encouraging results, the exact component(s) is yet to be elucidated. It is plausible that a myriad of components is present in the CC-Exos that can orchestrate cartilage regeneration including chondrogenesis and stability. However, the detailed mechanism of CC-Exos treatment to CPCs that caused the difference from that of BMSC-Exos is still unclear, and further investigation of RNA-seq is needed to dissect the components present in CC-Exos and to investigate their underlying mechanisms in cartilage repair. In addition, chondrogenesis is a complex process which is related to various signaling pathways, such as wnt, TGF-β, and hedgehog pathway. Here, we preliminarily demonstrated the induction of TGF-β and downstream SMAD2/3 expression after CC-Exos treatment. Further investigation is needed to acquire the entire picture of the pathway.
Conclusions
In summary, this study demonstrated that a novel exosome from chondrocytes could imitate the chondrogenic niche in a subcutaneous environment, which could facilitate chondrogenesis and maintenance of cartilage stability. This may contribute to its preferable chondroinductive niches coupled with its antiangiogenic properties. Thus, CC-Exos may represent a promising biologic-based therapeutic approach for the treatment of ectopic cartilage defects.
Abbreviations
- BMSC:
-
Bone mesenchymal stem cells
- BMSC-Exos:
-
Exosomes secreted by bone mesenchymal stem cells
- CC-Exos:
-
Exosomes secreted by chondrocytes
- COL II:
-
Collagen type II
- COL X:
-
Collagen type X
- CPCs:
-
Cartilage progenitor/stem cells
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- FBS:
-
Fetal bovine serum
- H&E:
-
Hematoxylin and eosin
- HUVECs:
-
Human umbilical vein endothelial cells
- IHH:
-
Indian hedgehog
- MMP 13:
-
Matrix metalloproteinase 13
- NC:
-
Negative control
- PBS:
-
Phosphate-buffered saline
- PC:
-
Positive control
- RT-PCR:
-
Real-time quantitative polymerase chain reaction
- SDF-1:
-
Stem cell-derived factor 1
- S-F:
-
Safranin-O/fast green
- T-B:
-
Toluidine blue
- TEM:
-
Transmission electron microscopy
- TGF-β:
-
Transforming growth factor-β
- VEGF:
-
Vascular endothelial growth factor
References
Liu X, Sun H, Yan D, Zhang L, Lv X, Liu T, et al. In vivo ectopic chondrogenesis of BMSCs directed by mature chondrocytes. Biomaterials. 2010;31:9406–14.
He A, **a H, **ao K, Wang T, Liu Y, Xue J, et al. Cell yield, chondrogenic potential, and regenerated cartilage type of chondrocytes derived from ear, nasoseptal, and costal cartilage. J Tissue Eng Regen Med. 2018;12:1123–32.
Kawanabe Y, Nagata S. A new method of costal cartilage harvest for total auricular reconstruction: part I. Avoidance and prevention of intraoperative and postoperative complications and problems. Plast Reconstr Surg. 2006;117:2011–8.
Padilla S, Sanchez M, Orive G, Anitua E. Human-based biological and biomimetic autologous therapies for musculoskeletal tissue regeneration. Trends Biotechnol. 2017;35:192–202.
Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338:917–21.
Johnstone B, Alini M, Cucchiarini M, Dodge GR, Eglin D, Guilak F, et al. Tissue engineering for articular cartilage repair – the state of the art. Eur Cells Mater. 2013;25:248–67.
Leijten JC, Georgi N, Wu L, van Blitterswijk CA, Karperien M. Cell sources for articular cartilage repair strategies: shifting from monocultures to cocultures. Tissue Eng Part B Rev. 2013;19:31–40.
Demoor M, Ollitrault D, Gomez-Leduc T, Bouyoucef M, Hervieu M, Fabre H, et al. Cartilage tissue engineering: molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochim Biophys Acta. 2014;1840:2414–40.
Shen W, Chen J, Zhu T, Chen L, Zhang W, Fang Z, et al. Intra-articular injection of human meniscus stem/progenitor cells promotes meniscus regeneration and ameliorates osteoarthritis through stromal cell-derived factor-1/CXCR4-mediated homing. Stem Cells Transl Med. 2014;3:387–94.
Mizuno M, Kobayashi S, Takebe T, Kan H, Yabuki Y, Matsuzaki T, et al. Brief report: reconstruction of joint hyaline cartilage by autologous progenitor cells derived from ear elastic cartilage. Stem Cells. 2014;32:816–21.
Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, Aigner T, Richter W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254–66.
Liu K, Zhou GD, Liu W, Zhang WJ, Cui L, Liu X, et al. The dependence of in vivo stable ectopic chondrogenesis by human mesenchymal stem cells on chondrogenic differentiation in vitro. Biomaterials. 2008;29:2183–92.
Becerra J, Santos-Ruiz L, Andrades JA, Mari-Beffa M. The stem cell niche should be a key issue for cell therapy in regenerative medicine. Stem Cell Rev. 2011;7:248–55.
Lane SW, Williams DA, Watt FM. Modulating the stem cell niche for tissue regeneration. Nat Biotechnol. 2014;32(8):795–803.
Jayasuriya CT, Chen Y, Liu W, Chen Q. The influence of tissue microenvironment on stem cell-based cartilage repair. Ann N Y Acad Sci. 2016;1383:21–33.
Leyh M, Seitz A, Dürselen L, Springorum H-R, Angele P, Ignatius A, et al. Osteoarthritic cartilage explants affect extracellular matrix production and composition in cocultured bone marrow-derived mesenchymal stem cells and articular chondrocytes. Stem Cell Res Ther. 2014;5:77.
Li D, Zhu L, Liu Y, Yin Z, Liu Y, Liu F, et al. Stable subcutaneous cartilage regeneration of bone marrow stromal cells directed by chondrocyte sheet. Acta Biomater. 2017;54:321–32.
Zhao X, Hwang NS, Bichara DA, Saris DB, Malda J, Vacanti JP, et al. Chondrogenesis by bone marrow-derived mesenchymal stem cells grown in chondrocyte-conditioned medium for auricular reconstruction. J Tissue Eng Regen Med. 2017;11:2763–73.
Ahmed N, Dreier R, Gopferich A, Grifka J, Grassel S. Soluble signalling factors derived from differentiated cartilage tissue affect chondrogenic differentiation of rat adult marrow stromal cells. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2007;20:665–678.
Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Semin Cell Dev Biol. 2015;40:82–8.
Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK, Toh WS. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27.
Katare R, Stroemer P, Hicks C, Stevanato L, Patel S, Corteling R, et al. Clinical-grade human neural stem cells promote reparative neovascularization in mouse models of hindlimb ischemia. Arterioscler Thromb Vasc Biol. 2014;34:408–18.
Jo W, Kim J, Yoon J, Jeong D, Cho S, Jeong H, et al. Large-scale generation of cell-derived nanovesicles. Nanoscale. 2014;6:12056–64.
Lee C, Carney RP, Hazari S, Smith ZJ, Knudson A, Robertson CS, et al. 3D plasmonic nanobowl platform for the study of exosomes in solution. Nanoscale. 2015;7:9290–7.
Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89.
Toh WS, Lai RC, Hui JHP, Lim SK. MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin Cell Dev Biol. 2017;67:56–64.
Melle ML d V–v, Narcisi R, Kops N, BSc WJLMK, et al. Chondrogenesis of mesenchymal stem cells in an osteochondral environment is mediated by the subchondral bone. Tissue Eng Part A. 2014;20:23–33.
Tao SC, Yuan T, Zhang YL, Yin WJ, Guo SC, Zhang CQ. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7:180–95.
Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noel D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 2017;7:16214.
Zhang S, Chu WC, Lai RC, Lim SK, Hui JH, Toh WS. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr Cartil. 2016;24:2135–40.
Xue K, **a W, Zhang X, Qi L, Zhou J, Xu P, Liu K. Isolation and identification of stem cells in different subtype of cartilage tissue. Expert Opin Biol Ther. 2015;15:623–32.
Dowthwaite GP, Bishop JC, Redman SN, Khan IM, Rooney P, Evans DJ, et al. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117:889–97.
Xue K, Qi L, Zhou G, Liu K. A two-step method of constructing mature cartilage using bone marrow-derived mesenchymal stem cells. Cells Tissues Organs. 2013;197:484–95.
Zhu Y, Wang Y, Zhao B, Niu X, Hu B, Li Q, et al. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res Ther. 2017;8:64.
Liu X, Wang S, Wu S, Hao Q, Li Y, Guo Z, Wang W. Exosomes secreted by adipose-derived mesenchymal stem cells regulate type I collagen metabolism in fibroblasts from women with stress urinary incontinence. Stem Cell Res Ther. 2018;9:159.
Wang X, Shen H, Zhangyuan G, Huang R, Zhang W, He Q, et al. 14-3-3zeta delivered by hepatocellular carcinoma-derived exosomes impaired anti-tumor function of tumor-infiltrating T lymphocytes. Cell Death Dis. 2018;9:159.
Arlov O, Steinwachs M, Skjak-Braek G, Zenobi-Wong M. Biomimetic sulphated alginate hydrogels suppress IL-1beta-induced inflammatory responses in human chondrocytes. Eur Cell Mater. 2017;33:76–89.
Chen CY, Rao SS, Ren L, Hu XK, Tan YJ, Hu Y, et al. Exosomal DMBT1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis. Theranostics. 2018;8:1607–23.
Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, et al. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells. 2015;33:2158–68.
Li Z, Ba R, Wang Z, Wei J, Zhao Y, Wu W. Angiogenic potential of human bone marrow-derived mesenchymal stem cells in chondrocyte brick-enriched constructs promoted stable regeneration of craniofacial cartilage. Stem Cells Transl Med. 2017;6:601–12.
Wang W, Qu M, Xu L, Wu X, Gao Z, Gu T, et al. Sorafenib exerts an anti-keloid activity by antagonizing TGF-beta/Smad and MAPK/ERK signaling pathways. J Mol Med (Berl). 2016;94:1181–94.
Sun JL, Jiao K, Niu LN, Jiao Y, Song Q, Shen LJ, et al. Intrafibrillar silicified collagen scaffold modulates monocyte to promote cell homing, angiogenesis and bone regeneration. Biomaterials. 2017;113:203–16.
Pan Y, Wu Q, Qin L, Cai J, Du B. Gold nanoparticles inhibit VEGF165-induced migration and tube formation of endothelial cells via the Akt pathway. Biomed Res Int. 2014;16:418624.
Leijten J, Georgi N, Moreira Teixeira L, van Blitterswijk CA, Post JN, Karperien M. Metabolic programming of mesenchymal stromal cells by oxygen tension directs chondrogenic cell fate. Proc Natl Acad Sci U S A. 2014;111:13954–9.
Chiang CS, Chen JY, Chiang MY, Hou KT, Li WM, Chang SJ, Chen SY. Using the interplay of magnetic guidance and controlled TGF-beta release from protein-based nanocapsules to stimulate chondrogenesis. Int J Nanomedicine. 2018;13:3177–88.
Chen MJ, Whiteley JP, Please CP, Schwab A, Ehlicke F, Waters SL, et al. Inducing chondrogenesis in MSC/chondrocyte co-cultures using exogenous TGF-beta: a mathematical model. J Theor Biol. 2018;439:1–13.
Yang J, Zhang YS, Yue K, Khademhosseini A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017;57:1–25.
Du Y, Liu H, Yang Q, Wang S, Wang J, Ma J, et al. Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials. 2017;137:37–48.
Kawanabe Y, Nagata S. A new method of costal cartilage harvest for total auricular reconstruction: part II. Evaluation and analysis of the regenerated costal cartilage. Plast Reconstr Surg. 2007;119:308–15.
Ou LF, Yan RS, Tang YW. Firm elevation of the auricle in reconstruction of microtia with a retroauricular fascial flap wrap** an autogenous cartilage wedge. Br J Plast Surg. 2001;54:573–80.
Dakak M, Gurkok S, Gozubuyuk A, Genc O. Surgical repair of congenital sternal cleft in an adult to prevent traumatic pericardial rupture. Thorac Cardiovasc Surg. 2006;54:551–3.
Del Frari B, Schwabegger AH. Diced autologous rib cartilage for primary treatment or refinement of minor chest wall deformities. Plast Reconstr Surg. 2011;128:154–62.
Haisch A, Klaring S, Groger A, Gebert C, Sittinger M. A tissue-engineering model for the manufacture of auricular-shaped cartilage implants. Eur Arch Otorhinolaryngol. 2002;259:316–21.
Huang BJ, Hu JC, Athanasiou KA. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials. 2016;98:1–22.
Brunelle AR, Horner CB, Low K, Ico G, Nam J. Electrospun thermosensitive hydrogel scaffold for enhanced chondrogenesis of human mesenchymal stem cells. Acta Biomater. 2018;6:166–76.
Patel JM, Ghodbane SA, Brzezinski A, Gatt CJ, Dunn MG. Tissue-engineered total meniscus replacement with a fiber-reinforced scaffold in a 2-year ovine model. Am J Sports Med. 2018;46:1844–56.
Poe AJ, Knowlton AA. Exosomes as agents of change in the cardiovascular system. J Mol Cell Cardiol. 2017;111:40–50.
Ribeiro-Rodrigues TM, Laundos TL, Pereira-Carvalho R, Batista-Almeida D, Pereira R, Coelho-Santos V, et al. Exosomes secreted by cardiomyocytes subjected to ischaemia promote cardiac angiogenesis. Cardiovasc Res. 2017;113:1338–50.
Li F, Truong VX, Fisch P, Levinson C, Glattauer V, Zenobi-Wong M, et al. Cartilage tissue formation through assembly of microgels containing mesenchymal stem cells. Acta Biomater. 2018;10:30409–4.
Liu C, Li T, Yang Z, Liu D, Li Y, Zhou Z, et al. Kartogenin enhanced chondrogenesis in cocultures of chondrocytes and bone mesenchymal stem cells. Tissue Eng Part A. 2018;24:990–1000.
Mumme M, Barbero A, Miot S, Wixmerten A, Feliciano S, Wolf F, et al. Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: an observational first-in-human trial. Lancet. 2016;388:1985–94.
Bhattacharjee M, Coburn J, Centola M, Murab S, Barbero A, Kaplan DL, et al. Tissue engineering strategies to study cartilage development, degeneration and regeneration. Adv Drug Deliv Rev. 2015;84:107–22.
El Andaloussi S, Mäger I, Breakefield XO, Wood MJA. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–57.
Jonitz A, Lochner K, Tischer T, Hansmann D, Bader R. TGF-beta1 and IGF-1 influence the re-differentiation capacity of human chondrocytes in 3D pellet cultures in relation to different oxygen concentrations. Int J Mol Med. 2012;30:666–72.
Lee SH, Che X, Jeong JH, Choi JY, Lee YJ, Lee YH, et al. Runx2 protein stabilizes hypoxia-inducible factor-1alpha through competition with von Hippel-Lindau protein (pVHL) and stimulates angiogenesis in growth plate hypertrophic chondrocytes. J Biol Chem. 2012;287:14760–71.
Leijten J, Georgi N, Moreira Teixeira L, van Blitterswijk CA, Post JN, et al. Metabolic programming of mesenchymal stromal cells by oxygen tension directs chondrogenic cell fate. PNAS. 2014;111:13954–9.
Acknowledgements
Not applicable.
Funding
This research was supported by the National Natural Science Foundation of China (81471878, 81272128, 30872697).
Availability of data and materials
All data generated or analyzed during this study are included in this published article. For additional information, please contact the author.
Author information
Authors and Affiliations
Contributions
YHC, ZWZ, and KL conceived the idea, designed the experiments, provided their funds for the study, and revised the manuscript. YHC and ZWZ designed the study and performed the research, data analysis, and manuscript writing. KX and XDZ contributed to the analyses and interpretation of data. All authors read and approved the final manuscript for publication.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All procedures of the animal experiments were approved by the Animal Research Committee of Shanghai Jiao Tong University Affiliated Ninth People’s Hospital (No. HKDL 2017-132).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Chen, Y., Xue, K., Zhang, X. et al. Exosomes derived from mature chondrocytes facilitate subcutaneous stable ectopic chondrogenesis of cartilage progenitor cells. Stem Cell Res Ther 9, 318 (2018). https://doi.org/10.1186/s13287-018-1047-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-018-1047-2