Abstract
Background
Diabetes mellitus is a devastating metabolic disease. Generation of insulin-producing cells (IPCs) from stem cells, especially from Wharton’s jelly mesenchymal stem cells (WJ-MSCs), has sparked much interest recently. Exendin-4 has several beneficial effects on MSCs and β cells. However, its effects on generation of IPCs from WJ-MSCs specifically have not been studied adequately. The purpose of this study was therefore to investigate how exendin-4 could affect the differentiation outcome of WJ-MSCs into IPCs, and to investigate the role played by exendin-4 in this differentiation process.
Methods
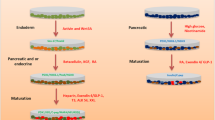
WJ-MSCs were isolated, characterized and then induced to differentiate into IPCs using two differentiation protocols: protocol A, without exendin-4; and protocol B, with exendin-4. Differentiated IPCs were assessed by the expression of various β-cell-related markers using quantitative RT-PCR, and functionally by measuring glucose-stimulated insulin secretion.
Results
The differentiation protocol B incorporating exendin-4 significantly boosted the expression levels of β-cell-related genes Pdx-1, Nkx2.2, Isl-1 and MafA. Moreover, IPCs generated by protocol B showed much better response to variable glucose concentrations as compared with those derived from protocol A, which totally lacked such response. Furthermore, exendin-4 alone induced early differentiation markers such as Pdx-1 and Nkx2.2 but not Isl-1, besides inducing late markers such as MafA. In addition, exendin-4 showed a synergistic effect with nicotinamide and β-mercaptoethanol in the induction of these markers.
Conclusions
Exendin-4 profoundly improves the differentiation outcome of WJ-MSCs into IPCs, possibly through the ability to induce the expression of β-cell markers.
Similar content being viewed by others
Background
Diabetes mellitus (DM) is a devastating metabolic disease associated with increased morbidity and mortality [1]. It is growing at an alarming rate, affecting more than 415 million people worldwide [2]. In DM, insulin-secreting β cells are damaged to different extents, leading to either absolute or relative insulin deficiency in type 1 and type 2 DM, respectively [1].
The transplantation of pancreatic islets has been demonstrated as a very effective treatment for DM, which could allow temporal insulin independence [3]. However, the availability of the donor islets could never meet the demand of the DM burden, which firmly establishes the clinical value of generating surrogate β cells from alternative renewable sources such as differentiation from stem cells [4].
Actually, within the last few years, various types of stem cells have been shown to be able to give rise to insulin-producing cells (IPCs), including embryonic stem cells (ESCs) [5, 6] and mesenchymal stem cells (MSCs) [7–9]. In fact, MSCs could indeed represent the stem cells of choice for regenerative medicine [10]. Among the various sources of MSCs, the umbilical cord (UC) together with other extra-embryonic tissues, which are routinely discarded at parturition, provide an untapped source of MSCs [11]. These sources do not impose any ethical concerns such as those which exist regarding ESCs, thus implying great potential for biomedical applications and cell-based therapeutic approaches [12].
The UC has been proved to be a good source of MSCs either from cord blood or cord tissue, also known as Wharton’s jelly (WJ) MSCs. The main fascination of WJ-MSCs lies in their possible banking, ease of isolation and large ex-vivo expansion capacity, as well as their demonstrated multipotency and immunomodulatory activities, which nominate them to become the new gold standard for MSC-based therapies [10]. Actually, in the context of diabetes research, WJ-MSCs sparked great interest [13] that is further encouraged by the recent interesting findings of multiple beneficial effects upon their injection into either diabetic patients or animals. Interestingly, Liu et al. applied WJ-MSC transplantation in type 2 DM patients, and demonstrated that treatment with WJ-MSCs can indeed improve metabolic control and β-cell function in patients with type 2 DM. They also suggested that their mechanism of action may have involved improvements in systemic inflammation [14]. Furthermore, another report highlighted the effect of intravenous infusion of human WJ-MSCs as a therapy by administering these cells in a type 2 DM rat model. The rats treated with WJ-MSCs exhibited increased numbers of β cells, suggesting the therapeutic potential of WJ-MSCs in β-cell regeneration [15]. Interestingly, our laboratory reported previously that WJ-MSCs exhibited better differentiation potential and control of hyperglycemia in streptozotocin-induced diabetic rats as compared with cord blood MSCs [16].
It is noteworthy here that there are several protocols used for generation of IPCs from MSCs in the literature. These protocols employ a huge variety of extrinsic factors [17, 18] and induction periods, which could vary from several days [9] to several months [17, Differentiation of WJ isolated cells into adipogenic, osteogenic and chondrogenic lineages We performed adipogenic, osteogenic and chondrogenic differentiation using the Human Mesenchymal Stem Cell Functional Identification Kit (R&D Systems Inc., MN, USA). The induction processes for the three lineages were performed according to the manufacturer’s instructions. Noninduced control WJ-MSCs were fed with complete growth medium (10 % FBS LG-DMEM) on the same schedule of each investigated lineage. Regarding adipogenic differentiation, after about 7 days lipid vacuoles started to appear in the induced cells. The detection of the resultant differentiated cells was carried out using Oil Red staining (Sigma-Aldrich, USA). For the osteogenic lineage, cells changed from spindle shaped to cuboidal shaped during differentiation, and differentiation was confirmed by Alizarin Red-S staining (Sigma-Aldrich, USA) for the calcium-rich extracellular matrix. Finally, regarding chondrogenic induction, cells changed from spindle shaped to cuboidal shaped during differentiation, and differentiation was confirmed by Alcian 8GX blue staining (Sigma-Aldrich, USA) for sulfated proteoglycan. After two to four passages, 1 × 106 WJ-MSCs were induced to differentiate into IPCs using two protocols. The first protocol (A) was carried out as described previously with slight modifications [9]; cells were preinduced for 48 hours with 10 mmol/L nicotinamide (Sigma-Aldrich, USA) and 1 mmol/L β-mercaptoethanol (Sigma-Aldrich, USA) in 10 % FBS LG-DMEM, and then reinduced for another 24 hours with 10 mmol/L nicotinamide and 1 mmol/L β-mercaptoethanol in serum-free high glucose (HG)-DMEM. The second protocol (B) started exactly as protocol A; the cells were preinduced for 48 hours with 10 mmol/L nicotinamide and 1 mmol/L β-mercaptoethanol in 10 % FBS LG-DMEM, and then reinduced for another 24 hours with 10 mmol/L nicotinamide and 1 mmol/L β-mercaptoethanol in serum-free HG-DMEM. However, this was followed by further induction for 7 days by 10 nmol/L exendin-4 (Sigma-Aldrich, USA) in serum-free HG-DMEM supplemented with 10 mmol/L nicotinamide and 1 mmol/L β-mercaptoethanol. Noninduced control WJ-MSCs were fed with complete growth medium (10 % FBS LG-DMEM) and kept for the same time as the differentiation protocol following the same culturing conditions as described earlier in this study. Briefly, WJ-MSCs were cultured for 10 days in 5 % FBS HG-DMEM, supplemented with either 10 nmol/L exendin-4 alone, 10 nmol/L exendin-4 and 10 mmol/L nicotinamide, or 10 nmol/L exendin-4 together with 10 mmol/L nicotinamide and 1 mmol/L β-mercaptoethanol. Noninduced control WJ-MSCs were fed with complete growth medium (10 % FBS LG-DMEM) and kept for the same time as the induced cells following the same culturing conditions as described earlier in this study. RNA extraction and quantitative reverse transcriptase PCR (qRT-PCR) analyses were then performed on the resulting cells for various β-cell markers. Both control undifferentiated WJ-MSCs and differentiated IPCs (resulting from both protocols) were collected. RNA was isolated using TRIzol Reagent (Life Technologies, USA) according to the manufacturer’s instructions. Briefly, 3 × 106 cells were treated by 1 ml TRIzol followed by extraction using chloroform and isopropanol. The cDNA was prepared by the Verso™ cDNA synthesis kit (Thermo Scientific, USA) using 0.5 μg RNA. Each qRT-PCR reaction was performed using 4 ng cDNA using the SYBR Green Master Mix (Applied Biosystems, USA). GAPDH was used as an internal control. ΔΔCt was used to calculate relative expression levels. RNA expression of various pancreatic development markers was measured by qRT-PCR. Forward and reverse primers for target genes are presented in Table 1. All qRT-PCR analyses were carried out using Step-One plus qRT-PCR (Applied Biosystems, USA). The maturity of differentiated IPCs was assessed by its ability to secrete insulin in response to high glucose challenge. Briefly, the differentiated cells were washed twice with PBS and Krebs Ringer bicarbonate (KRB) buffer, and then incubated for 1 hour in KRB buffer supplemented with 5.5 mM glucose in 37 °C, 5 % CO2 humidified atmosphere. Afterwards, cells were incubated with 5.5 mM glucose for 1 hour and then with 16.7 mM glucose in KRB buffer in the same conditions for 1 hour, and the supernatant was collected at the end of each incubation and frozen at –80 °C until the time of the assay. Insulin release was detected by the Accubind® insulin enzyme-linked immunosorbent assay (Monobind Inc., CA, USA) according to the manufacturer’s instructions. About 1 × 105 cells/well were seeded in six-well culture plates and exposed to various differentiation protocols. At the end of each differentiation protocol, Dithizone (DTZ) staining was performed to confirm IPC differentiation as described previously [30]. A stock solution of DTZ was prepared as follows: 50 mg of DTZ (Sigma Aldrich, USA) was completely dissolved in 5 ml of dimethyl sulfoxide (DMSO) and stored at –20 °C in the dark. For staining, a working solution was prepared by diluting the stock solution 1:100 in culture medium and then filtered through a 0.2-μm nylon filter. Then 3 ml of DTZ working solution was added to each well and incubated for at least 30 minutes at 37 °C. The cells were then carefully washed three times by PBS. Crimson red-stained IPCs were observed under an inverted phase-contrast microscope. Uninduced WJ-MSCs cultured in complete growth medium (LG-DMEM supplemented with 10 % FBS) were used as control. Data are presented as mean ± standard error of mean. Comparisons between the groups were conducted using a t test. For experiments with more than two groups comparison between means was conducted using one-way ANOVA, and the Bonferroni post-hoc test was applied to compare individual groups. All statistical analyses were carried out using the Windows-based SPSS statistical package (SPSS version 17.0; SPSS, Chicago, IL, USA). p < 0.05 was considered significant.Pancreatic endocrine differentiation
Effect of exendin-4 alone and in combination with other extrinsic factors on β-cell markers
RNA extraction and real-time RT-PCR analysis
Functional assessment of differentiated cells by glucose challenge test for insulin release (GSIS assay)
Dithizone staining
Statistical analyses
Results
WJ is a potential source of MSCs exhibiting all MSC characteristics
Adherent cells with fibroblast-like morphology could be observed as early as 10–14 days post plating of the explants of UC WJ. As shown in Fig. 1a, these cells were almost homogeneous, resembling MSC morphology. These cells showed excellent culture properties. Furthermore, these isolated fibroblast-like cells exhibit all of the MSC characteristics defined by the International Society for Cellular Therapy (ISCT) [31]. First, they were plastic adherent. Second, we performed flow cytometry for MSCs and hematopoietic specific cluster of differentiation (CD) markers. As shown in Fig. 1b, immunophenoty** revealed that isolated cells were negative for CD34 (hematopoietic stem cells) and CD14 (monocytes); the percentage of cells expressing these CDs did not exceed 5 %. On the contrary, they were positive for MSC markers CD73, CD90 and CD105, exhibiting expression intensities of 99.2 %, 99.9 % and 98.6 % respectively for these markers. These results indicate a relatively homogeneous mesenchymal phenotypic population of WJ-MSCs.
a Phase-contrast image of isolated WJ-MSCs (passage 3) showing homogeneous fibroblast-like cells. Magnification: 10×; scale bar = 100 μm. b Immunophenoty** for isolated WJ-MSCs; cells were labeled with FITC-conjugated or PE-conjugated antibodies and examined by flow cytometry. The immunophenoty** profile of WJ-MSCs showed negative for CD34 and CD14 but positive for CD73, CD90 and CD105. c–h Differentiation of isolated cells down several mesenchymal lineages: c uninduced WJ-MSCs as control for adipogenic differentiation; d induced WJ-MSCs showing red staining of oil droplets using Oil Red, characteristic for successful adipogenic differentiation; e uninduced control WJ-MSCs for osteogenic differentiation experiment; f induced WJ-MSCs showing positive Alizarin Red-S staining for calcium-rich extracellular matrix, indicating successful osteogenic differentiation characteristic for MSCs; g uninduced control WJ-MSCs for the chondrogenic differentiation experiment; h induced WJ-MSCs showing blue staining by Alcian 8GX blue for sulfated proteoglycan, indicating the successful chondrogenic differentiation of isolated WJ-MSCs. Scale bar = 100 μm
As a functional assay to further confirm the MSC identity of isolated cells, we examined the differentiation potential of these cells towards mesenchymal lineages. As shown in Fig. 1c–h, these WJ-MSCs exhibit adipogenic differentiation potential; detected by Oil Red staining of lipid droplets in comparison with control undifferentiated cells. Furthermore, these cells show an osteogenic differentiation potential; detected by Alizarin Red-S staining for calcium-rich extracellular matrix as compared with their control uninduced counterparts. Finally, the chondrogenic differentiation potential of the isolated WJ-MSCs was detected by Alcian 8GX blue staining for sulfated proteoglycan in comparison with control uninduced cells.
Exendin-4 improves the differentiation outcome of WJ-MSCs to IPCs
For generation of IPCs from WJ-MSCs, we tried two protocols: protocol A without exendin-4; and protocol B with exendin-4. As shown in Fig. 2a–d, at the end of both protocols the cells tended to lose their fibroblast-like shape and formed aggregates. Furthermore, some cells started to detach and grew as a suspension in culture media. However, control undifferentiated cells for both protocols kept their fibroblast-like morphology throughout the whole protocol period. Interestingly, when staining the resulting putative IPCs with DTZ, the resulting differentiated cells from both protocols were positively stained by DTZ as shown in Fig. 2e–h.
a–d Phase-contrast images of differentiated IPCs obtained from WJ-MSCs after induction by differentiation protocols: upon differentiation, cells lose their fibroblastic morphology and tend to aggregate forming clusters, which tend to detach and grow in suspension media, in contrast to their control WJ-MSCs which retain fibroblast-like morphology. Magnification, 20×; scale bar = 20 μm. e–h Phase-contrast images of generated IPCs stained by Dithizone (DTZ): e uninduced WJ-MSCs as a control showing negative DTZ staining; f IPCs generated by protocol A showing positive crimson red staining by DTZ; g uninduced WJ-MSCs as a control showing negative DTZ staining; h IPCs generated by protocol B showing positive crimson red staining by DTZ. Magnification 40×; scale bar = 20 μm. i In-vitro GSIS assay for differentiated IPCs derived from protocol A (without exendin-4) and protocol B (with exendin-4); insulin release in response to either 5.5 mM or 16.7 mM glucose concentrations was measured after 1 hour of incubation. It is noteworthy that control uninduced undifferentiated WJ-MSCs did not show any detectable levels of secreted insulin in response to either 5.5 mM or 16.7 mM glucose concentrations. Results presented as mean ± standard error of mean, obtained from three independent experiments. *Means are significantly different from secreted insulin levels in response to 5.5 mM glucose at p < 0.05. GSIS glucose-stimulated insulin secretion, HG high glucose, LG low glucose
To functionally assess the IPCs generated with these protocols, we performed glucose-stimulated insulin secretion (GSIS) for the derived IPCs. As shown in Fig. 2i, IPCs generated by protocol A did not show any response to variable glucose concentrations (LG 5.5 mM: 5.3 ± 0.12 μIU/ml secreted insulin, HG 16.7 mM: 5.43 ± 0.01 μIU/ml secreted insulin, p = 0.339). However, IPCs generated by protocol B showed higher secretion of insulin in response to HG as compared with LG concentrations (LG 5.5 mM: 7.45 ± 0.3 μIU/ml secreted insulin, HG 16.7 mM: 11.36 ± 1.23 μIU/ml secreted insulin, p = 0.037). So far, these results indicate better differentiation of IPCs generated by protocol B. Moreover, we examined the expression of Pdx-1 in differentiated IPCs generated from WJ-MSCs using the two protocols by immunocytochemistry. As shown in Additional file 1: Figure S1 (uppermost panel), control undifferentiated cells obviously lacked Pdx-1 expression. Upon exposure of these cells to protocol A (middle panel) they failed to express Pdx-1, indicating failure of this protocol to induce proper differentiation. On the contrary, using protocol B (lowest panel) the cells showed expression of Pdx-1, indicating differentiation of these cells into IPCs.
In spite of similar morphological changes, the mRNA expression levels of β-cell-related genes were quite different between these protocols. As shown in Fig. 3a–d, the induced cells derived from protocol A showed relatively no elevation at all for Pdx-1 expression, a slight modest elevation of the expression levels of Nkx2.2 and Isl-1, and elevation of MafA transcript levels. However, for protocol B the induced cells showed a profound significant elevation for the expression levels of Pdx-1, Nkx2.2, Isl-1 and MafA, as compared with their control uninduced counterparts.
Gene expression analyses by qRT-PCR for several β-cell markers. a Pdx-1, b Nkx2.2, c Isl-1 and d MafA in differentiated IPCs derived from differentiation protocols A and B as compared with their control uninduced WJ-MSCs. Results are presented as mean ± standard error of mean, obtained from three independent experiments. *Means are significantly different from control uninduced WJ-MSCs at p < 0.05
Exendin-4 alone can induce Pdx-1, Nkx2.2 and MafA but not Isl-1, while exhibiting a synergistic effect with nicotinamide and β-mercaptoethanol
To gain more insight into the role played by exendin-4 alone and in combination with other extrinsic factors on the differentiation markers of WJ-MSCs into IPCs, we incubated the cells with exendin-4 alone and in combination with nicotinamide alone or nicotinamide plus β-mercaptoethanol. As shown in Fig. 4a, exendin-4 alone induced the Pdx-1 expression level. This elevation was further induced by adding nicotinamide and β-mercaptoethanol to significant levels as compared with the control group. Also, as shown in Fig. 4b, exendin-4 showed an induction of Nkx2.2 transcript level as compared with the control group. However, in contrast to Pdx-1, addition of nicotinamide and β-mercaptoethanol did not show any further elevation in the expression levels of this transcription factor. Interestingly, Fig. 4c clearly illustrated that exendin-4 alone failed to induce the expression of Isl-1, while adding nicotinamide and β-mercaptoethanol with exendin-4 induced increased expression levels of Isl-1. Again, as shown in Fig. 4d, the MafA expression level showed a slight elevation with exendin-4 alone. However, addition of nicotinamide and β-mercaptoethanol with exendin-4 significantly induced further elevation of MafA levels as compared with the control group.
Gene expression analyses by qRT-PCR for cells induced by individual extrinsic factors, namely exendin-4 alone (Ex), exendin-4 plus nicotinamide (Ex + NA), and exendin-4 plus nicotinamide and β-mercaptoethanol (Ex + NA + BME), in 5 % FBS HG-DMEM. Gene expression analyses were done for several β-cell-related genes, namely a Pdx-1, b Nkx2.2, c Isl-1 and d MafA presented as relative mRNA expression levels in induced cells relative to their uninduced control counterparts. Results presented as mean ± standard error of mean for three independent experiments. *Means are significantly different from control at p < 0.05. #Means are significantly different from exendin-4 alone (Ex) at p < 0.05
Discussion
In the current study, we isolated WJ-MSCs and investigated their differentiation into IPCs using two differentiation protocols; with or without exendin-4. Our results showed that WJ-MSCs indeed fulfilled all MSC characteristics, and showed good potential to differentiate into IPCs. Additionally, exendin-4 was found to have profound beneficial effects on the differentiation outcome of those WJ-MSCs into IPCs. Besides, although our results showed that exendin-4 alone can induce the expression of some β-cell markers, namely Pdx-1, Nkx2.2 and MafA but not Isl-1, there still exists a synergistic effect on the induction of such markers upon addition of nicotinamide and β-mercaptoethanol with overall improvement of differentiation outcome.
Strategies to create surrogate β cells for therapeutic replacement have ignited significant excitement over the last decade, which has fueled profound interest in deriving functioning IPCs from stem cells [32]. Our results regarding the observed ease of isolation and relatively homogeneous population of isolated WJ-MSCs came in accordance with all of the reports highlighting the important potential of WJ-MSCs for various regenerative medicine applications [28].
Despite all efforts so far regarding the differentiation of stem cells to IPCs, current protocols are not optimized for different reasons, including the pleotropic effects induced by individual morphogens, together with the complexity of the signaling pathways involved and differences between the various types of used stem cells [18]. Further exploration into the differentiation of several stem cells to IPCs is thus indeed warranted together with investigating the various effects of incorporated inducing factors in the differentiation process [17, 33].
In the current study, we investigated two differentiation protocols, namely protocol A (without exendin-4) and protocol B (with exendin-4). Actually, both protocols induced the cells to detach and form clusters, and both of them showed positive DIZ staining; no apparent morphological differences were thus observed in IPCs generated from both protocols. Interestingly, the IPCs derived from protocol B, with exendin-4, showed a profound induction for the expression levels of all investigated β-cell-related genes in the current study, Pdx-1, Nkx2.2, Isl-1 and MafA, as compared with their control uninduced WJ-MSCs. These beneficial effects were further extended to our observations in the GSIS assay in which IPCs generated by protocol B demonstrated a reasonable response to HG as compared with LG concentrations, while those generated from protocol A totally lacked such response. Moreover, when carrying out immunocytochemistry to detect Pdx-1 in IPCs generated by both protocols, those generated by protocol B clearly expressed Pdx-1 but those derived from protocol A did not show such clear expression. Those findings also imply failure of protocol A lacking exendin-4 to induce proper differentiation, and further highlight the beneficial effects of exendin-4 on the differentiation outcome. Moreover, exendin-4 could enhance the viability of the generated IPCs as shown in Additional file 1: Figure S2. Actually, this might be attributed to the beneficial effects which have been reported recently for exendin-4 regarding improving MSC proliferation and cell survival [ Our results show that WJ-MSCs represent a readily available, noninvasive, highly promising source of stem cells for β-cell replacement therapies. Although the abundance of literature suggests that generation of IPCs from stem cells is feasible, many considerations such as the cell source, induction protocols and mechanisms of differentiation should be further explored before the application of these cells to clinical settings for treatment of DM. Most importantly, the principal novel finding of the current study is the beneficial enhancing effects of exendin-4 for the differentiation of WJ-MSCs into IPCs. In addition, this study shows that exendin-4 could induce early and late markers along the differentiation process. Finally, these findings open the door for further warranted investigations to gain more in-depth understanding of some hidden parts of the story of differentiation of stem cells into IPCs, and the mechanisms of action of various extrinsic factors. These hidden parts, if unraveled, will certainly help to resolve the difficulties of obtaining mature functioning IPCs. CD, cluster of differentiation; DIZ, Dithizone; DM, diabetes mellitus; DMEM, Dulbecco’s modified Eagle’s medium; ESC, embryonic stem cell; FITC, fluroisothiocyanate; GSIS, glucose-stimulated insulin secretion; HG, high glucose; IPC, insulin-producing cell; ISCT, International Society for Cellular Therapy; Isl-1, insulin gene enhancer protein (ISLET-1); KRB, Krebs Ringer bicarbonate; LG, low glucose; MafA, v-maf avian musculoaponeurotic fibrosarcoma oncogene homolog A; MSC, mesenchymal stem cell; NKx2.2, homeobox protein Nkx2.2; Pdx-1, pancreatic and duodenal homeobox 1; PE, phycoerythrin; qRT-PCR, quantitative reverse transcriptase PCR; UC, umbilical cord; WJ, Wharton’s jellyConclusions
Abbreviations
References
Harcourt B, Penfold S, Forbes J. Coming full circle in diabetes mellitus: from complications to initiation. Nat Rev Endocrinol. 2013;9(2):113–23.
IDF. International Diabetes Federation. IDF Diabetes Atlas. 7th ed. 2015. http://www.diabetesatlas.org/resources/2015-atlas.html. Accessed 1 Apr 2016.
Shapiro A, Lakey J, Ryan E, Korbutt G, Toth E, Warnock G, Kneteman N, Rajotte R. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–8.
Bruin JE, Rezania A, Kieffer TJ. Replacing and safeguarding pancreatic β cells for diabetes. Sci Transl Med. 2015;7(316):316 ps323.
Pagliuca F, Millman J, Gurtler M, Segel M, Van Dervort A, Ryu J, Peterson Q, Greiner D, Melton D. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159(2):428–39.
Rezania A, Bruin J, Arora P, Rubin A, Batushansky I, Asadi A, O'Dwyer S, Quiskamp N, Mojibian M, Albrecht T, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotech. 2014;32(11):1121–33.
Wu X, Liu C, Xu K, Mao X, Zhu J, Jiang J, Cui D, Zhang M, Xu Y, Liu C. Reversal of hyperglycemia in diabetic rats by portal vein transplantation of islet-like cells generated from bone marrow mesenchymal stem cells. World J Gastroenterol. 2007;13(24):3342–9.
Chao K, Chao K, Fu Y, Liu S. Islet-like clusters derived from mesenchymal stem cells in Wharton’s Jelly of the human umbilical cord for transplantation to control type 1 diabetes. PLoS One. 2008;3(1):e1451.
Chen L, Jiang X, Yang L. Differentiation of rat marrow mesenchymal stem cells into pancreatic islet beta-cells. World J Gastroenterol. 2004;10(20):3016–20.
El Omar R, Beroud J, Stoltz J, Menu P, Velot E, Decot V. Umbilical cord mesenchymal stem cells: the new gold standard for mesenchymal stem cell based therapies? Tissue Eng Part B Rev. 2014;20:523–44.
La Rocca G, Anzalone R, Corrao S, Magno F, Loria T, Lo Iacono M, Di Stefano A, Giannuzzi P, Marasà L, Cappello F, et al. Isolation and characterization of Oct-4+/HLA-G+ mesenchymal stem cells from human umbilical cord matrix: differentiation potential and detection of new markers. Histochem Cell Biol. 2009;131(2):267–82.
Bhonde R, Sheshadri P, Sharma S, Kumar A. Making surrogate β-cells from mesenchymal stromal cells: perspectives and future endeavors. Int J Biochem Cell Biol. 2014;46:90–102.
Anzalone R, Lo Iacono M, Loria T, Di Stefano A, Giannuzzi P, Farina F, La Rocca G. Wharton’s jelly mesenchymal stem cells as candidates for beta cells regeneration: extending the differentiative and immunomodulatory benefits of adult mesenchymal stem cells for the treatment of type 1 diabetes. Stem Cell Rev. 2011;7(2):342–63.
Liu X, Zheng P, Wang X, Dai G, Cheng H, Zhang Z, Hua R, Niu X, Shi J, An Y. A preliminary evaluation of efficacy and safety of Wharton’s jelly mesenchymal stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cell Res Ther. 2014;5(2):57.
Hu J, Wang F, Sun R, Wang Z, Yu X, Wang L, Gao H, Zhao W, Yan S, Wang Y. Effect of combined therapy of human Wharton’s jelly-derived mesenchymal stem cells from umbilical cord with sitagliptin in type 2 diabetic rats. Endocrine. 2014;45(2):279–87.
El-Demerdash RF, Hammad LN, Kamal MM, El Mesallamy HO. A comparison of Wharton’s jelly and cord blood as a source of mesenchymal stem cells for diabetes cell therapy. Regen Med. 2015;10(7):841–55.
Wong R. Extrinsic factors involved in the differentiation of stem cells into insulin-producing cells: an overview. Exp Diabetes Res. 2011;2011:15.
Champeris T, Jones P. Generating pancreatic β-cells from embryonic stem cells by manipulating signaling pathways. J Endocrinol. 2010;206(1):13–26.
Tang D, Cao L, Burkhardt B, **a C, Litherland S, Atkinson M, Yang L. In vivo and In vitro characterization of insulin-producing cells obtained from murine bone marrow. Diabetes. 2004;53(7):1721–32.
Hrvatin S, O'Donnell C, Deng F, Millman J, Pagliuca F, DiIorio P, Rezania A, Gifford D, Melton D. Differentiated human stem cells resemble fetal, not adult, β-cells. Proc Natl Acad Sci U S A. 2014;11(8):3038–43.
Thorens B, Porret A, Bühler L, Deng S, Morel P, Widmann C. Cloning and functional expression of the human islet GLP-1 receptor: demonstration that exendin-4 is an agonist and exendin-(9–39) an antagonist of the receptor. Diabetes. 1993;42(11):1678–82.
Li H, Lam A, Xu A, Sl Lam K, Kim Chung S. High dosage of exendin-4 increased early insulin secretion in differentiated beta cells from mouse embryonic stem cells. Acta Pharmacol Sin. 2010;31(5):570–7.
Gabr MM, Sobh MM, Zakaria MM, Refaie AF, Ghoneim MA. Transplantation of insulin-producing clusters derived from adult bone marrow stem cells to treat diabetes in rats. Exp Clin Transplant. 2008;6(3):236–43.
Phuc P, Nhung T, Loan D, Chung D, Ngoc P. Differentiating of banked human umbilical cord blood-derived mesenchymal stem cells into insulin-secreting cells. In Vitro Cell Dev Biol Anim. 2011;47(1):54–63.
Eng J, Kleinman W, Singh L, Singh G, Raufman J. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem. 1992;267(11):7402–5.
Xu G, Stoffers D, Habener J, Bonner-Weir S. Exendin-4 stimulates both β-cell replication and neogenesis, resulting in increased β-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48(12):2270–6.
Fong C, Chak L, Biswas A, Tan J, Gauthaman K, Chan W, Bongso A. Human Wharton’s jelly stem cells have unique transcriptome profiles compared to human embryonic stem cells and other mesenchymal stem cells. Stem Cell Rev Rep. 2011;7(1):1–16.
Kalaszczynska I, Ferdyn K. Wharton’s jelly derived mesenchymal stem cells: future of regenerative medicine? Recent findings and clinical significance. BioMed Res Int. 2015;2015:11.
Petsa A, Gargani S, Felesakis A, Grigoriadis N, Grigoriadis I. Effectiveness of protocol for the isolation of Wharton’s Jelly stem cells in large-scale applications. In Vitro Cell Dev Biol Anim. 2009;45(10):573–6.
Shiroi A, Yoshikawa M, Yokota H, Fukui H, Ishizaka S, Tatsumi K, Takahashi Y. Identification of insulin-producing cells derived from embryonic stem cells by zinc-chelating dithizone. Stem Cells. 2002;20(4):284–92.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7.
Pagliuca F, Melton D. How to make a functional β-cell. Development. 2013;140(12):2472–83.
Dave S. Extrinsic factors promoting insulin producing cell-differentiation and insulin expression enhancement-hope for diabetics. Curr Stem Cell Res Ther. 2013;8(6):471–83.
Zhou H, Li D, Shi C, **n T, Yang J, Zhou Y, Hu S, Tian F, Wang J, Chen Y. Effects of Exendin-4 on bone marrow mesenchymal stem cell proliferation, migration and apoptosis in vitro. Sci Rep. 2015;5:12898.
Khorsandi L, Saremy S, Khodadadi A, Dehbashi F. Effects of exendine-4 on the differentiation of insulin producing cells from rat adipose-derived mesenchymal stem cells. Cell J (Yakhteh). 2016;17(4):720–9.
Nejad-Dehbashi F, Hashemitabar M, Orazizadeh M, Bahramzadeh S, Shahhosseini Pourshoushtary E, Khorsandi L. The effects of exendine-4 on insulin producing cell differentiation from rat bone marrow-derived mesenchymal stem cells. Cell J (Yakhteh). 2014;16(2):187–94.
Lee Y, Jun H. Anti-diabetic actions of glucagon-like peptide-1 on pancreatic beta-cells. Metabolism. 2014;63(1):9–19.
Timper K, Seboek D, Eberhardt M, Linscheid P, Christ-Crain M, Keller U, Muller B, Zulewski H. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem Biophys Res Commun. 2006;341(4):1135–40.
Kodama S, Toyonaga T, Kondo T, Matsumoto K, Tsuruzoe K, Kawashima J, Goto H, Kume K, Kume S, Sakakida M, et al. Enhanced expression of PDX-1 and Ngn3 by exendin-4 during beta cell regeneration in STZ-treated mice. Biochem Biophys Res Commun. 2005;327(4):1170–8.
Hui H, Perfetti R. Pancreas duodenum homeobox-1 regulates pancreas development during embryogenesis and islet cell function in adulthood. Eur J Endocrinol. 2002;146(2):129–41.
Kubo A, Stull R, Takeuchi M, Bonham K, Gouon-Evans V, Sho M, Iwano M, Saito Y, Keller G, Snodgrass R. Pdx1 and Ngn3 overexpression enhances pancreatic differentiation of mouse ES cell-derived endoderm population. PLoS One. 2011;6(9):e24058.
Shiroi A, Ueda S, Ouji Y, Saito K, Moriya K, Sugie Y, Fukui H, Ishizaka S, Yoshikawa M. Differentiation of embryonic stem cells into insulin-producing cells promoted by Nkx2.2 gene transfer. World J Gastroenterol. 2005;11(27):4161–6.
Henseleit KD, Nelson SB, Kuhlbrodt K, Hennings JC, Ericson J, Sander M. NKX6 transcription factor activity is required for alpha- and beta-cell development in the pancreas. Development. 2005;132(13):3139–49.
May CL. The role of Islet-1 in the endocrine pancreas: lessons from pancreas specific Islet-1 deficient mice. Islets. 2010;2(2):121–3.
Artner I, Hang Y, Mazur M, Yamamoto T, Guo M, Lindner J, Magnuson MA, Stein R. MafA and MafB regulate genes critical to beta-cells in a unique temporal manner. Diabetes. 2010;59(10):2530–9.
El Khattabi I, Sharma A. Proper activation of MafA is required for optimal differentiation and maturation of pancreatic beta-cells. Best Pract Res Clin Endocrinol Metab. 2015;29(6):821–31.
**n Y, Jiang X, Wang Y, Su X, Sun M, Zhang L, Tan Y, Wintergerst KA, Li Y. Insulin-producing cells differentiated from human bone marrow mesenchymal stem cells in vitro ameliorate streptozotocin-induced diabetic hyperglycemia. PLoS One. 2016;11(1):e0145838.
Acknowledgements
The authors would like to thank Dr Rabab El-Hawary, Department of Clinical and Chemical Pathology, Faculty of Medicine, Cairo University, Cairo, Egypt for kindly providing antibodies and hel** with the flowcytometry experiments.
Funding
This study was personally funded.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Authors’ contributions
HOE and MMK conceived and designed the experiments. MMK and DHK performed the experiments. AGE, MMK and DHK provided and processed the UC samples. HOE, MMK and DHK analyzed the data. HOE, MMK and DHK wrote the article. All authors reviewed the manuscript. All authors read and approved the manuscript.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
This study was approved by the ethical committees of both the Faculty of Pharmacy and the Faculty of Medicine, Ain Shams University. The UCs were obtained from the Gynecology and Obstetrics Department, Ain Shams University Hospitals, from both cesarean section and normal labor after obtaining signed informed consent from the parents.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1: Figure S1.
Immunocytochemistry of control undifferentiated cells (upper most panel) and differentiated IPCs generated by both protocols; protocol A (middle panel) and protocol B (lowest panel) for Pdx-1 (red). The nuclei were stained with DAPI (blue), scale bar = 100 μm. Figure S2. Viability assay for control WJ-MSCs, differentiated IPCs in presence of exendin-4 and differentiated IPCs in absence of exendin-4. *significantly different from control at p < 0.05, #significantly different from exendin-4 group at p < 0.05. (DOCX 186 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kassem, D.H., Kamal, M.M., El-Kholy, A.EL.G. et al. Exendin-4 enhances the differentiation of Wharton’s jelly mesenchymal stem cells into insulin-producing cells through activation of various β-cell markers. Stem Cell Res Ther 7, 108 (2016). https://doi.org/10.1186/s13287-016-0374-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-016-0374-4