Abstract
Background
Published methods for quantification of magnetic resonance imaging (MRI) evidence of inflammation in the sacroiliac joint lack validation in pediatric populations. We evaluated the reliability and construct validity of the Spondyloarthritis Research Consortium of Canada (SPARCC) sacroiliac joint inflammation score (SIS) in children with suspected or confirmed juvenile spondyloarthritis (JSpA).
Methods
The SPARCC SIS measures the presence, depth, and intensity of bone marrow inflammation on MRI through the cartilaginous part of the joint. Six readers blinded to clinical details except age, participated in two reading exercises, each preceded by a calibration exercise. Inter-observer reliability was assessed using intraclass correlation coefficients (ICCs) and for pre-specified acceptable reliability the inraclass correlation coefficient (ICC) was > 0.8.
Results
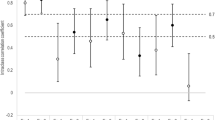
The SPARCC SIS had face validity and was feasible to score in pediatric cases in both reading exercises. Cases were mostly male (64%) and the median age at the time of imaging was 14.9 years. After calibration, the median ICC across all readers for the SIS total score was 0.81 (IQR 0.71–0.89). SPARCC SIS had weak correlation with disease activity (DA) as measured by the JSpADA (r = − 0.12) but discriminated significantly between those with and without elevated C-reactive protein (p = 0.03).
Conclusion
The SPARCC SIS was feasible to score and had acceptable reliability in children. The ICC improved with additional calibration and reading exercises, for both experienced and inexperienced readers.
Similar content being viewed by others
Background
Children with spondyloarthritis (SpA) and axial arthritis are at risk of progression to ankylosing spondylitis, an inflammatory disease that causes joint fusion and leads to permanent functional impairment. While there are similarities between juvenile and adult SpA, there are distinct phenotypic differences that warrant specific focus and clinical trials in juvenile disease [1,2,3,11] that have not been validated in children. We did not see correlation with disease activity measures, perhaps secondary to the relatively small sample size or the lack of validated specific measures of axial disease activity to use as reference standards. One measure specifically designed to assess clinical disease activity in juvenile SpA is the JSpADA index [15], but this is an assessment of overall SpA disease activity and axial disease activity accounts for only two of eight variables contributing to the score. No other validated juvenile arthritis disease activity tools assess axial disease activity. The SPARCC SIS did discriminate between those with and without elevated C-reactive protein. This is not entirely surprising given the results from the aforementioned adult studies [11] and the pediatric studies demonstrating increased predictive probability of sacroiliitis with elevated C-reactive protein [4]. Discrimination was not uniform across multiple clinical attributes as it did not discriminate those with and without back pain. This latter finding may be a problem unique to pediatric SpA in which back pain has been shown to be poorly correlated with the presence of axial arthritis in multiple studies [4, 16].
There are several limitations to our study that should be considered. First, the number of pediatric studies was limited, largely due to missing semi-coronal sequences which are essential for SPARCC scoring. The limited number of studies necessitated re-evaluation during the second exercise of some cases used in the first reading exercise. We do not believe using the same studies more than once impacted our results because the cases were not discussed amongst the readers after the first reading exercise and the two reading exercises were separated by approximately 15 months. The limited number of studies also means we could not assess the minimal detectable difference or change in scores; this will be a focus of future work. Second, the studies utilized in the reading and calibration exercises were primarily from only two North American hospitals. Data collection from only two hospitals frequently leads to overrepresentation of regional demographics and a cohort that may not be typical of the entire disease population; however, this limitation is mitigated by the fact that both of these institutions are large referral centers, so the studies included are likely to be representative of cases evaluated in a wide variety of geographic areas. Third, some physician and patient-reported assessments were missing, which is expected in a retrospective study. Fourth, specific clinical measures of axial disease activity were not available as they are neither validated nor routinely collected in pediatric clinical care. Although these latter two issues limited the assessment of construct and discriminative validity, they do not affect the feasibility or reliability assessment. These relatively minor limitations are to be expected in the first systematic assessment of the feasibility and reliability of a new tool. Fifth, there was no gold standard by which to assess construct validity of the tool. The closest constructs to measure clinical (not radiographic) disease activity were the juvenile SpA disease activity index and the physician global assessment of disease activity. At most, we hypothesized there would be modest correlation. The absence a current gold standard should not preclude assessment of the feasibility and reliability utility of the tool.
Conclusions
We have demonstrated the feasibility and reliability of the SPARCC sacroiliac joint inflammation scoring methodology in children with established or suspected spondyloarthritis. This scoring system is based upon dichotomous scoring of lesions on consecutive slices through the cartilaginous part of the sacroiliac joint. We have not only established feasibility but also demonstrated that inexperienced readers can have their reading calibrated using standardized definitions, DICOM reference cases and an interactive calibration module. Further work is needed to assess the responsiveness and prognostic significance of the SPARCC SIS in evaluating MRI lesions in children.
Abbreviations
- ASAS :
-
Assessment of SpA International Society
- CRP:
-
C-reactive protein
- DICOM :
-
Digital Imaging and Communication in Medicine
- ICC :
-
Intraclass correlation coefficients
- JSpA :
-
Juvenile spondyloarthritis
- JSpADA :
-
Jjuvenile SpA disease activity index
- MRI :
-
magnetic resonance imaging
- SIS :
-
sacroiliac joint inflammation score
- SpA :
-
spondyloarthritis
- SPARCC :
-
Spondyloarthritis Research Consortium of Canada
- STIR :
-
Short-tau inversion recovery
- TNF :
-
Tumor necrosis factor
References
Jadon DR, Shaddick G, Jobling A, Ramanan AV, Sengupta R. Clinical outcomes and progression to orthopedic surgery in juvenile- versus adult-onset ankylosing spondylitis. Arthritis Care Res. 2015;67(5):651–7.
Lin YC, Liang TH, Chen WS, Lin HY. Differences between juvenile-onset ankylosing spondylitis and adult-onset ankylosing spondylitis. J Chin Med Assoc. 2009;72(11):573–80.
Gensler LS, Ward MM, Reveille JD, Learch TJ, Weisman MH, Davis JC Jr. Clinical, radiographic and functional differences between juvenile-onset and adult-onset ankylosing spondylitis: results from the PSOAS cohort. Ann Rheum Dis. 2008;67(2):233–7.
Weiss PF, **ao R, Biko DM, Chauvin NA. Assessment of sacroiliitis at diagnosis of juvenile spondyloarthritis by radiography, magnetic resonance imaging, and clinical examination. Arthritis Care Res. 2016;68(2):187–94.
O'Shea FD, Boyle E, Riarh R, Tse SM, Laxer RM, Inman RD. Comparison of clinical and radiographic severity of juvenile-onset versus adult-onset ankylosing spondylitis. Ann Rheum Dis. 2009;68(9):1407–12.
Horneff G, Fitter S, Foeldvari I, Minden K, Kuemmerle-Deschner J, Tzaribacev N, Thon A, Borte M, Ganser G, Trauzeddel R, et al. Double-blind, placebo-controlled randomized trial with adalimumab for treatment of juvenile onset ankylosing spondylitis (JoAS): significant short term improvement. Arthritis Res Ther. 2012;14(5):R230.
Lukas C, Landewe R, Sieper J, Dougados M, Davis J, Braun J, van der Linden S, van der Heijde D. Assessment of SpondyloArthritis international S. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis. 2009;68(1):18–24.
Althoff CE, Sieper J, Song IH, Haibel H, Weiss A, Diekhoff T, Rudwaleit M, Freundlich B, Hamm B, Hermann KG. Active inflammation and structural change in early active axial spondyloarthritis as detected by whole-body MRI. Ann Rheum Dis. 2013;72(6):967–73.
Maksymowych WP, Lambert RG, Brown LS, Pangan AL. Defining the minimally important change for the SpondyloArthritis Research Consortium of Canada Spine and sacroiliac joint magnetic resonance imaging indices for ankylosing spondylitis. J Rheumatol. 2012;39(8):1666–74.
Pedersen SJ, Poddubnyy D, Sorensen IJ, Loft AG, Hindrup JS, Thamsborg G, Asmussen K, Hendricks O, Norregaard J, Piil AD, et al. Course of magnetic resonance imaging-detected inflammation and structural lesions in the sacroiliac joints of patients in the randomized, double-blind, placebo-controlled Danish multicenter study of adalimumab in spondyloarthritis, as assessed by the Berlin and Spondyloarthritis Research Consortium of Canada Methods. Arthritis Rheum. 2016;68(2):418–29.
Maksymowych WP, Dougados M, van der Heijde D, Sieper J, Braun J, Citera G, Van den Bosch F, Logeart I, Wajdula J, Jones H, et al. Clinical and MRI responses to etanercept in early non-radiographic axial spondyloarthritis: 48-week results from the EMBARK study. Ann Rheum Dis. 2016;75(7):1328–35.
Lambert RG, Salonen D, Rahman P, Inman RD, Wong RL, Einstein SG, Thomson GT, Beaulieu A, Choquette D, Maksymowych WP. Adalimumab significantly reduces both spinal and sacroiliac joint inflammation in patients with ankylosing spondylitis: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2007;56(12):4005–14.
Sieper J, van der Heijde D, Dougados M, Mease PJ, Maksymowych WP, Brown MA, Arora V, Pangan AL. Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomised placebo-controlled trial (ABILITY-1). Ann Rheum Dis. 2013;72(6):815–22.
Sieper J, van der Heijde D, Dougados M, Maksymowych WP, Scott BB, Boice JA, Berd Y, Bergman G, Curtis S, Tzontcheva A, et al. A randomized, double-blind, placebo-controlled, sixteen-week study of subcutaneous golimumab in patients with active nonradiographic axial spondyloarthritis. Arthritis Rheum. 2015;67(10):2702–12.
Weiss PF, Colbert RA, **ao R, Feudtner C, Beukelman T, DeWitt EM, Pagnini I, Wright TB, Wallace CA. Development and retrospective validation of the juvenile spondyloarthritis disease activity index. Arthritis Care Res. 2014;66(12):1775–82.
Stoll ML, Bhore R, Dempsey-Robertson M, Punaro M. Spondyloarthritis in a pediatric population: risk factors for sacroiliitis. J Rheumatol. 2010;37(11):2402–8.
Acknowledgements
Dr Jaremko and Dr Lambert are supported by Medical Imaging Consultants, Edmonton.
Funding
Dr Weiss’ work was supported by the Rheumatology Research Foundation. Dr Jaremko is supported by the Capital Health Chair in Diagnostic Imaging.
Availability of data and materials
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosures
Dr. Weiss has served as a consultant for Lilly.
Author information
Authors and Affiliations
Contributions
PW designed the study, participated in the reading exercise, analyzed the results and drafted the manuscript. WPM contributed to the design of the study, participated in the reading exercise and interpretation of the results and helped revise the manuscript. RGL participated in the reading exercise, interpretation of the results and manuscript revision. JLJ participated in the reading exercise, interpretation of the results and manuscript revision. DMB participated in the reading exercise, interpretation of the results and manuscript revision. JP was responsible for feasibility and coordination of data acquisition and participated in data analysis. TGB participated in study design, coordination, data analysis, interpretation and drafting of the manuscript. RX participated in data analysis and drafting of the manuscript. NAC participated in the reading exercise, interpretation of the results and manuscript revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study’s protocols were reviewed and approved by the Children’s Hospital of Philadelphia Committee for the Protection of Human Subjects (16–012641 and 17–013883). Waivers of consent and HIPAA authorization were granted as the procedures represented minimal risk to the subjects and did not adversely affect the rights and welfare of the subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Weiss, P.F., Maksymowych, W.P., Lambert, R.G. et al. Feasibility and reliability of the Spondyloarthritis Research Consortium of Canada sacroiliac joint inflammation score in children. Arthritis Res Ther 20, 56 (2018). https://doi.org/10.1186/s13075-018-1543-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-018-1543-x