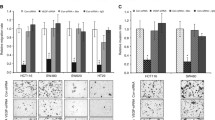
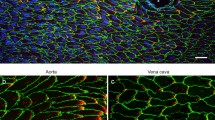
Abstract
Endothelial cell migration is a key process in angiogenesis. Progress of endothelial cell migration is orchestrated by coordinated generation of Ca2+ signals through a mechanism organized in caveolar microdomains. Connexins (Cx) play a central role in coordination of endothelial cell function, directly by cell-to-cell communication via gap junction and, indirectly, by the release of autocrine/paracrine signals through Cx-formed hemichannels. However, Cx hemichannels are also permeable to Ca2+ and Cx43 can be associated with caveolin-1, a structural protein of caveolae. We proposed that endothelial cell migration relies on Cx43 hemichannel opening. Here we show a novel mechanism of Ca2+ signaling in endothelial cell migration. The Ca2+ signaling that mediates endothelial cell migration and the subsequent tubular structure formation depended on Cx43 hemichannel opening and is associated with the translocation of Cx43 with caveolae to the rear part of the cells. These findings indicate that Cx43 hemichannels play a central role in endothelial cell migration and provide new therapeutic targets for the control of deregulated angiogenesis in pathological conditions such as cancer.
Similar content being viewed by others
Introduction
Tissue functional integrity and viability relies on the accurate delivery of oxygen and nutrients through the blood flow, according to the cellular activity along the time. Although regulation of blood flow distribution through dynamic control of vasomotor tone of resistance arteries in the microcirculation can timely match the changes in cellular metabolic demand, the vessel density and the microvascular network architecture must be coherent with the physiological function of the tissue. In this context, long-term regulation of tissue irrigation depends on the growth of new blood vessels from pre-existing ones through a process known as angiogenesis [1, 2], which is stimulated by tissue requirements of oxygen as observed in different physiological and pathological conditions such as wound healing, tissue regeneration, embryonic development, and tumor growth [3].
Angiogenesis is a complex process that involves a cascade of events initiated by coordinated endothelial cell migration [4] in response to an increase in intracellular Ca2+ concentration ([Ca2+]i). Ca2+ has been recognized as a key signaling mechanism in angiogenesis [5, 6] and although Ca2+ release from intracellular stores plays an important role in endothelial cell migration, the prevalence of the response depends on Ca2+ entry from the extracellular compartment. However, the mechanisms involved in this process are controversial and have not been clearly determined [7,8,9]. In addition, it has been proposed that cell migration also depends on the intercellular coordination of Ca2+ signals and, consistent with this, blockade of gap junctions results in a reduction in both migration and tubular structure formation by endothelial cells [10, 11].
Gap junctions are intercellular channels that directly connect the cytoplasm of adjacent cells, allowing the exchange of current, ions and small signaling molecules (< 1.4 nm of diameter), such as second messengers. These intercellular channels are made up by serial docking of two hemichannels, each one provided by each neighboring cell and, in turn, hemichannels are formed by the assembly of six connexin (Cx) proteins [12]. It should be noted, however, that, in addition to form gap junctions, individual hemichannels can also be functional [13, 14]. Thus, the opening of these channels contributes to the transmembrane communication of the intra- and extracellular compartments [15, 16], which may be relevant in the control of endothelial cell function, since Cx43 hemichannels have been reported to be activated by nitric oxide (NO) through direct S-nitrosylation of this Cx protein [Endothelial cell-mediated formation of tubular structures The analysis of endothelial cells tube formation was performed in 12 mm coverslips covered with 100 µl Matrigel® (Corning, NY, USA) according to the manufacturer’s protocol. Matrigel®-solution was added to coverslips located into a 96 wells plate and allowed to solidify and polymerize at 37ºC in a 5% CO2-95% air atmosphere. Then, endothelial cells were seeded on top of the Matrigel and tubular-like structure formation was evaluated for 6 and 12 h in control conditions or in the presence of 300 µM TAT-Gap19. Seven fields per coverslip were examined using a Nikon Eclipse E600 FN1 microscope and the results were expressed as the angiogenic index according to the following formula: (Total cells + connected cells)/ total cells x (1-non-connected cells), where Total cells is the number of total cell in the field, connected cells is the number of the cells that form a tubular structures and non-connected cells is the number of the cells outside of tubular structures. Results are expressed as mean ± SEM. All values represent data from at least three independent cultures. Comparison between groups was performed using unpaired or paired Student t-test, one-way ANOVA followed by Bonferroni post-hoc test or two-ways ANOVA as appropriate. P < 0.05 was considered significant.Statistical analysis
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 2011;12:551–64. https://doi.org/10.1038/nrm3176.
Betsholtz C. Cell-cell signaling in blood vessel development and function. EMBO Mol. 2018. https://doi.org/10.15252/emmm.201708610. medicine10, e8610.
Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003. https://doi.org/10.1038/nm0603-653. 6:653 – 60.
Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res100. 2007;782–94. https://doi.org/10.1161/01.RES.0000259593.07661.1e.
Moccia F, Negri S, Shekha M, Faris P, Guerra G. Endothelial Ca2+ signaling, angiogenesis and vasculogenesis: just what it takes to make a blood vessel. Int J Mol. 2019;20. https://doi.org/10.3390/ijms20163962. Sci.
Munaron L, Fiorio Pla A. Endothelial calcium machinery and angiogenesis: understanding physiology to interfere with pathology. Curr Med Chem. 2009;16:4691–703. https://doi.org/10.2174/092986709789878210.
Banumathi E, O’Connor A, Gurunathan S, Simpson DA, McGeown JG, Curtis TM. (2011) VEGF-induced retinal angiogenic signaling is critically dependent on Ca2+ signaling by Ca2+/calmodulin-dependent protein kinase II. Invest Ophthalmol Vis Sci. 11;52(6):3103-11. https://doi.org/10.1167/iovs.10-6574.
Faehling M, Kroll J, Föhr KJ, Fellbrich G, Mayr U, Trischler G, Waltenberger J. Essential role of calcium in vascular endothelial growth factor A-induced signaling: mechanism of the antiangiogenic effect of carboxyamidotriazole. FASEB J. 2002;16:1–29. https://doi.org/10.1096/fj.01-0938fje.
Kohn EC, Alessandro R, Spoonster J, Wersto RP, Liotta LA. Angiogenesis: role of calcium-mediated signal transduction. Proc Natl Acad Sci USA. 1995;92(5):1307–11. https://doi.org/10.1073/pnas.92.5.1307.
Gärtner C, Ziegelhöffer B, Kostelka M, Stepan H, Mohr FW, Dhein S. Knock-down of endothelial connexins impairs angiogenesis. Pharmacol Res. 2012;65(3):347–57. https://doi.org/10.1016/j.phrs.2011.11.012.
Okamoto T, Akita N, Kawamoto E, Hayashi T, Suzuki K, Shimaoka M. Endothelial connexin32 enhances angiogenesis by positively regulating tube formation and cell migration. Exp Cell Res. 2014;321(2):133–41. https://doi.org/10.1016/j.yexcr.2013.12.002.
Evans WH, Martin PE. Gap junctions: structure and function (review). Mol Membr Biol. 2002;19(2):121–36. https://doi.org/10.1080/09687680210139839.
De Smet MA, Lissoni A, Nezlobinsky T, Wang N, Dries E, Pérez-Hernández M, Lin X, Amoni M, Vervliet T, Witschas K, Rothenberg E, Bultynck G, Schulz R, Panfilov AV, Delmar M, Sipido KR, Leybaert L. Cx43 hemichannel microdomain signaling at the intercalated disc enhances cardiac excitability. J Clin Invest. 2021;131(7):e137752. https://doi.org/10.1172/JCI137752.
De Bock M, De Smet M, Verwaerde S, Tahiri H, Schumacher S, Van Haver V, Witschas K, Steinhäuser C, Rouach N, Vandenbroucke RE, Leybaert L. Targeting gliovascular connexins prevents inflammatory blood-brain barrier leakage and astrogliosis. JCI Insight. 2022;7(16):e135263. https://doi.org/10.1172/jci.insight.135263.
Evans WH, De Vuyst E, Leybaert L. The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem J. 2006;397(1):1–14. https://doi.org/10.1042/BJ20060175.
De Bock M, Wang N, Bol M, Decrock E, Ponsaerts R, Bultynck G, Dupont G, Leybaert L. Connexin 43 hemichannels contribute to cytoplasmic Ca2 + oscillations by providing a bimodal Ca2+-dependent Ca2+ entry pathway. J Biol Chem. 2012;287(15):12250–66. https://doi.org/10.1074/jbc.M111.299610.
Lillo MA, Himelman E, Shirokova N, **e LH, Fraidenraich D, Contreras JE. S-nitrosylation of connexin43 hemichannels elicits cardiac stress-induced arrhythmias in Duchenne muscular dystrophy mice. JCI Insight. 2019;4(24):e130091. https://doi.org/10.1172/jci.insight.130091.
Retamal MA, Cortés CJ, Reuss L, Bennett MV, Sáez JC. S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. Proc Natl Acad Sci U S A. 2006;103(12):4475–80. https://doi.org/10.1073/pnas.0511118103.
Berra-Romani R, Avelino-Cruz JE, Raqeeb A, Della Corte A, Cinelli M, Montagnani S, Guerra G, Moccia F, Tanzi F. Ca2+-dependent nitric oxide release in the injured endothelium of excised rat aorta: a promising mechanism applying in vascular prosthetic devices in aging patients. BMC Surg. 2013;13(Suppl 2):40. https://doi.org/10.1186/1471-2482-13-S2-S40.
Braet K, Paemeleire K, D’Herde K, Sanderson MJ, Leybaert L. Astrocyte-endothelial cell calcium signals conveyed by two signalling pathways. Eur J Neurosci. 2001;13(1):79–91. https://doi.org/10.1111/j.1460-9568.2001.01372.x.
Bol M, Wang N, De Bock M, Wacquier B, Decrock E, Gadicherla A, Decaluwé K, Vanheel B, van Rijen HV, Krysko DV, Bultynck G, Dupont G, Van de Voorde J, Leybaert L. At the cross-point of connexins, calcium, and ATP: blocking hemichannels inhibits vasoconstriction of rat small mesenteric arteries. Cardiovasc Res. 2017;113(2):195–206. https://doi.org/10.1093/cvr/cvw215.
Figueroa XF, Lillo MA, GaetePS, Riquelme MA, Saez JC. Diffusion of nitric oxide across cell membranes of the vascular wall requires specific connexin-based channels. Volume 75. Neuropharmacology; 2013. pp. 471–8. https://doi.org/10.1016/j.neuropharm.2013.02.022.
Wei C, Wang X, Zheng M, Cheng H. Calcium gradients underlying cell migration. Curr Opin Cell Biol. 2012;24(2):254–61. https://doi.org/10.1016/j.ceb.2011.12.002.
Vinken M, Decrock E, De Vuyst E, Ponsaerts R, D’hondt C, Bultynck G, Ceelen L, Vanhaecke T, Leybaert L, Rogiers V. Connexins: sensors and regulators of cell cycling. Biochim Biophys Acta. 2011;1815(1):13–25. https://doi.org/10.1016/j.bbcan.2010.08.004.
Koepple C, Zhou Z, Huber L, Schulte M, Schmidt K, Gloe T, Kneser U, Schmidt VJ, de Wit C. Expression of Connexin43 stimulates endothelial angiogenesis independently of gap Junctional Communication in Vitro. Int J Mol Sci. 2021;22(14):7400. https://doi.org/10.3390/ijms22147400.
Zhou Z, Chai W, Liu Y, Zhou M, Zhang X. Connexins and angiogenesis: functional aspects, pathogenesis, and emerging therapies (review). Int J Mol Med. 2022;50(2):110. https://doi.org/10.3892/ijmm.2022.5166.
Shi W, Meng Z, Luo J. Connexin 43 (Cx43) regulates high-glucose-induced retinal endothelial cell angiogenesis and retinal neovascularization. Front Endocrinol (Lausanne). 2022;13:909207. https://doi.org/10.3389/fendo.2022.909207.
Mannell H, Kameritsch P, Beck H, Pfeifer A, Pohl U, Pogoda K. Cx43 promotes endothelial cell Migration and Angiogenesis via the Tyrosine phosphatase SHP-2. Int J Mol Sci. 2021;23(1):294. https://doi.org/10.3390/ijms23010294.
Abudara V, Bechberger J, Freitas-Andrade M, De Bock M, Wang N, Bultynck G, Naus CC, Leybaert L, Giaume C. The connexin43 mimetic peptide Gap19 inhibits hemichannels without altering gap junctional communication in astrocytes. Front Cell Neurosci. 2014;8:306. https://doi.org/10.3389/fncel.2014.00306.
Walrave L, Pierre A, Albertini G, Aourz N, De Bundel D, Van Eeckhaut A, Vinken M, Giaume C, Leybaert L, Smolders I. (2018) Inhibition of astroglial connexin43 hemichannels with TAT-Gap19 exerts anticonvulsant effects in rodents. Glia66(8):1788–1804. https://doi.org/10.1002/glia.23341.
De Bock M, Culot M, Wang N, Bol M, Decrock E, De Vuyst E, Leybaert L. Connexin channels provide a target to manipulate brain endothelial calcium dynamics and blood-brain barrier permeability. J Cereb Blood Flow Metab. 2011;31(9):1942–57. https://doi.org/10.1038/jcbfm.2011.86.
Evans WH, Boitano S. Connexin mimetic peptides: specific inhibitors of gap-junctional intercellular communication. Biochem Soc Trans. 2001;29(Pt4):606–12. https://doi.org/10.1042/bst0290606.
Suarez S, Ballmer-Hofer K. VEGF transiently disrupts gap junctional communication in endothelial cells. J Cell Sci. 2001;114(Pt 6):1229–35. https://doi.org/10.1242/jcs.114.6.1229.
Okamoto T, Usuda H, Tanaka T, Wada K, Shimaoka M. The functional implications of endothelial gap Junctions and Cellular mechanics in vascular angiogenesis. Cancers (Basel). 2019;11(2):237. https://doi.org/10.3390/cancers11020237.
De Bock M, Wang N, Bol M, Decrock E, Ponsaerts R, Bultynck G, Dupont G, Leybaert L. Connexin 43 hemichannels contribute to cytoplasmic Ca2 + oscillations by providing a bimodal Ca2+-dependent Ca2 + entry pathway. J Biol Chem. 2012;287(15):12250–66. https://doi.org/10.1074/jbc.M111.299610.
Berra-Romani R, Raqeeb A, Avelino-Cruz JE, Moccia F, Oldani A, Speroni F, Tanzi F. (2008). Ca2+ signaling in injured in situ endothelium of rat aorta.Cell calcium. 44(3):298–309. https://doi.org/10.1016/j.ceca.2007.12.007.
Noren DP, Chou WH, Lee SH, Qutub AA, Warmflash A, Wagner DS, Popel AS, Levchenko A. Endothelial cells decode VEGF-mediated Ca2 + signaling patterns to produce distinct functional responses. Sci Signal. 2016;9(416):ra20. https://doi.org/10.1126/scisignal.aad3188.
Michaelis UR. Mechanisms of endothelial cell migration. Cell Mol Life Sci. 2014;71(21):4131–48. https://doi.org/10.1007/s00018-014-1678-0.
Pani B, Singh BB. Lipid rafts/caveolae as microdomains of calcium signaling. Cell Calcium. 2009;45(6):625–33. https://doi.org/10.1016/j.ceca.2009.02.009.
Isshiki M, Anderson RGW. Function of Caveolae in Ca2+ entry and Ca2+-Dependent Signal Transduction. Traffic. 2003;4(11):717–23. https://doi.org/10.1034/j.1600-0854.2003.00130.x.
Grande-García A, Echarri A, de Rooij J, Alderson NB, Waterman-Storer CM, Valdivielso JM, del Pozo MA. Caveolin-1 regulates cell polarization and directional migration through src kinase and rho GTPases. J Cell Biol. 2007;177(4):683–94. https://doi.org/10.1083/jcb.200701006.
Sun X, Liu Z, Chen H, Beardsley A, Qi Q, Liu J. A conserved sequence in caveolin-1 is both necessary and sufficient for caveolin polarity and cell directional migration. FEBS Lett. 2009;583(22):3681–9. https://doi.org/10.1016/j.febslet.2009.10.055.
Isshiki M, Ando J, Yamamoto K, Fujita T, Ying Y, Anderson RG. Sites of ca(2+) wave initiation move with caveolae to the trailing edge of migrating cells. J Cell Sci115(Pt. 2002;3475–84. https://doi.org/10.1242/jcs.115.3.475.
Sáez JC, Contreras-Duarte S, Gómez GI, Labra VC, Santibañez CA, Gajardo-Gómez R, Avendaño BC, Díaz EF, Montero TD, Velarde V, Orellana JA. Connexin 43 Hemichannel Activity promoted by pro-inflammatory cytokines and high glucose alters endothelial cell function. Front Immunol. 2018. https://doi.org/10.3389/fimmu.2018.01899.
Delvaeye T, De Smet MAJ, Verwaerde S, Decrock E, Czekaj A, Vandenbroucke RE, Lemeire K, Gonçalves A, Declercq W, Vandenabeele P, Krysko DV, Leybaert L. Blocking connexin43 hemichannels protects mice against tumour necrosis factor-induced inflammatory shock. Sci Rep. 2019;9(1):16623. https://doi.org/10.1038/s41598-019-52900-4.
Devika NT, Jaffar Ali BM. Analysing calcium dependent and independent regulation of eNOS in endothelium triggered by extracellular signalling events. Mol Biosyst. 2013;9(11):2653–64. https://doi.org/10.1039/C3MB70258H.
Ungvari Z, Sun D, Huang A, Kaley G, Koller A. Role of endothelial [Ca2+]i in activation of eNOS in pressurized arterioles by agonists and wall shear stress. Am J Physiol Heart Circ Physiol. 2001;281(2):H606–12. https://doi.org/10.1152/ajpheart.2001.281.2.H606.
Forrester M, Foster MW, Benhar M, Stamler JS. Detection of protein S-nitrosylation with the biotin-switch technique. Free Radic Biol Med. 2009;46(2):119–26. https://doi.org/10.1016/j.freeradbiomed.2008.09.034.
Figueroa XF, Poblete MI, Boric MP, Mendizábal VE, Adler-Graschinsky E, Huidobro-Toro JP. Clonidine-induced nitric oxide-dependent vasorelaxation mediated by endothelial alpha(2)-adrenoceptor activation. Br J Pharmacol. 2001;134(5):957–68. https://doi.org/10.1038/sj.bjp.0704320.
Ashley RA, Dubuque SH, Dvorak B, Woodward SS, Williams SK, Kling PJ. Erythropoietin stimulates vasculogenesis in neonatal rat mesenteric microvascular endothelial cells. Pediatr Res. 2002;51:472–8. https://doi.org/10.1203/00006450-200204000-00012.
Funding
This work was supported by Grant Anillo ANID/ACT210057 from the Agencia Nacional de Investigación y Desarrollo, Grant #1150530 from Fondo Nacional de Desarrollo Científico y Tecnológico – FONDECYT and by PhD and Thesis support scholarship #21170977 from Comisión Nacional de Investigación Científica y Tecnológica – CONICYT.
Author information
Authors and Affiliations
Contributions
Hilda Espinoza. Collected and analyzed the data, prepared figures and participated in the manuscript preparation. Xavier Figueroa. Directed and supervised the investigation, designed the experimental protocols, contributed to data analysis and manuscript writing and editing.
Corresponding author
Ethics declarations
Ethical approval
All studies and experimental procedures were approved by the Institutional Bioethics Committee (protocol ID 170823033).
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Espinoza, H., Figueroa, X.F. Opening of Cx43-formed hemichannels mediates the Ca2+ signaling associated with endothelial cell migration. Biol Direct 18, 52 (2023). https://doi.org/10.1186/s13062-023-00408-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13062-023-00408-3