Abstract
Background
Extracorporeal membrane oxygenation (ECMO) has been used extensively for coronavirus disease 2019 (COVID-19)-related acute respiratory distress syndrome (ARDS). Reports early in the pandemic suggested that mortality in patients with COVID-19 receiving ECMO was comparable to non-COVID-19-related ARDS. However, subsequent reports suggested that mortality appeared to be increasing over time. Therefore, we conducted an updated systematic review and meta-analysis, to characterise changes in mortality over time and elucidate risk factors for poor outcomes.
Methods
We conducted a meta-analysis (CRD42021271202), searching MEDLINE, Embase, Cochrane, and Scopus databases, from 1 December 2019 to 26 January 2022, for studies reporting on mortality among adults with COVID-19 receiving ECMO. We also captured hospital and intensive care unit lengths of stay, duration of mechanical ventilation and ECMO, as well as complications of ECMO. We conducted random-effects meta-analyses, assessed risk of bias of included studies using the Joanna Briggs Institute checklist and evaluated certainty of pooled estimates using GRADE methodology.
Results
Of 4522 citations, we included 52 studies comprising 18,211 patients in the meta-analysis. The pooled mortality rate among patients with COVID-19 requiring ECMO was 48.8% (95% confidence interval 44.8–52.9%, high certainty). Mortality was higher among studies which enrolled patients later in the pandemic as opposed to earlier (1st half 2020: 41.2%, 2nd half 2020: 46.4%, 1st half 2021: 62.0%, 2nd half 2021: 46.5%, interaction p value = 0.0014). Predictors of increased mortality included age, the time of final patient enrolment from 1 January 2020, and the proportion of patients receiving corticosteroids, and reduced duration of ECMO run.
Conclusions
The mortality rate for patients receiving ECMO for COVID-19-related ARDS has increased as the pandemic has progressed. The reasons for this are likely multifactorial; however, as outcomes for these patients evolve, the decision to initiate ECMO should include the best contextual estimate of mortality at the time of ECMO initiation.
Similar content being viewed by others
Introduction
Extracorporeal membrane oxygenation (ECMO) has been used extensively for coronavirus disease 2019 (COVID-19)-related acute respiratory distress syndrome (ARDS). However, it is highly resource intensive, leading to challenges in provision during the pandemic [1]. A systematic review and meta-analysis examining patients who received ECMO for COVID-19 in 2020 reported a 37% mortality rate [2]. As the pandemic progressed, treatment practices and patterns evolved, and newer variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged. Alongside these changes, contemporaneous studies reported increasing mortality rates and longer duration of ECMO runs in patients with COVID-19 ARDS. The mortality rate reported by the Extracorporeal Life Support Organisation (ELSO) registry data for the use of ECMO in COVID-19 increased from 37% in early 2020 to 52% by the end of 2020 [3, 4], demonstrating the dynamic nature of clinical outcomes during the course of the pandemic.
While subsequent single-centre studies have shown similar trends, the mortality rates for patients receiving ECMO for COVID-19 appear variable globally, with reports of rates ranging from 17.5% to 68% in the first 18 months of the pandemic [5]. Several reasons related to patient, disease, and treatment factors have been postulated for this and include increased virulence of SARS-CoV-2 variants [5, 6]; changes in patient selection patterns based, at times, on local resource availability; changes in interventions, including the need of using prolonged noninvasive forms of mechanical ventilation and delays in endotracheal intubation due to the overwhelming number of patients with respiratory failure; and the use of immunomodulators such as corticosteroids and interleukin-6 receptor antagonists [3, 7]. Based on this, we performed an updated systematic review and meta-analysis to summarise outcome data during the first 2 years of the pandemic, including the changes in mortality trends, and identify risk factors for unfavourable outcomes in order to guide clinical decision-making and further research.
Methodology
Search strategy and selection criteria
We registered the protocol with PROSPERO (CRD42021271202) and conducted the review in adherence with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Statement (Additional file 1: Table S1) [8]. We searched MEDLINE, Embase, Cochrane and Scopus databases from 1 December 2019 to 26 January 2022 using the following keywords and their variations: “extracorporeal membrane oxygenation”, “extracorporeal life support”, “SARS-CoV-2” and “COVID-19” (Additional file 1: Table S2). We also reviewed the reference lists of included studies and review articles on the topic. We included studies or online registries reporting on at least 10 adult patients with COVID-19 requiring ECMO. We excluded any studies primarily reporting on animals or paediatric patients (< 16 years old). In the case of overlap** patient data, we included the largest study and excluded any other overlap** studies.
Data collection and risk of bias assessment
We collected data using a prespecified data extraction form. Authors were contacted for additional data where necessary (Additional file 1: Table S3). We assessed individual study risk of bias using the appropriate Joanna Briggs Institute checklist for case series or cohort studies. We assessed certainty of evidence using the Grading of Recommendations, Assessments, Developments and Evaluations (GRADE) approach [9]. The screening of studies, data collection, and risk of bias assessment were conducted independently and in duplicate by RRL and JJLS, and FA assisted with the risk of bias assessment. Conflicts were resolved by consensus or by KR. Where there was missing data, we contacted the corresponding authors of each study to obtain additional data for analysis.
Data synthesis
The primary outcome was mortality at the longest recorded time of follow-up. Secondary outcomes included ICU and hospital and length of stay, duration of invasive mechanical ventilation, duration of ECMO, and complications during ECMO (which we then classified according to the broad groups described by ELSO). We performed random-effects meta-analyses (DerSimonian and Laird) based on the logit transformation [10,11,12], and computed 95% confidence intervals (CIs) using the Clopper–Pearson method [13]. As inter-study heterogeneity in observational studies tends to be overestimated by I2 statistics, we assessed statistical heterogeneity (inconsistency) as part of the GRADE approach [9], using I-squared but also the Chi-squared test and visual inspection of the forest plots [14]. We assessed for publication bias qualitatively using visual inspection of funnel plots, and quantitatively using Egger’s regression test. We corrected for small-study effects using the random-effects trim-and-fill (R0 estimator) procedure. As some centres which published studies on their patient cohort report that patient data to the ELSO registry, there is a risk of duplicating patient data when including studies reporting on data from the ELSO registry. Hence, we conducted a sensitivity analysis excluding any studies reporting on ELSO registry data. We also conducted a second analysis excluding studies with high risks of bias (defined as JBI score < 7) and analysed the mortality among studies specifically reporting on outcomes of patients receiving venovenous ECMO (VV-ECMO). We present survival outcomes as pooled proportions, while continuous outcomes are presented as pooled means, both with corresponding 95% CIs.
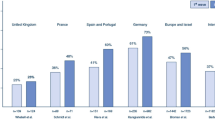
We conducted pre-specified subgroup analysis based on the geographical region (North America, Latin America, Asia–Pacific, Europe, Southwest Asia and Africa), as well as by time period (every six months from 1 January 2020, defined by the date of enrolment of the last patient included in each study). We conducted univariable meta-regression when at least 6 data points were reported, to explore potential sources of heterogeneity, or prognostically relevant prespecified study-level covariates (date of last patient enrolment [per 100 days from 1 January 2020], age [per year], proportion of male patients, and patients receiving corticosteroids and interleukin blockers [percentage], body mass index [per 1 kg/m2], SOFA score [every increase by 1 point], PaO2/FiO2 ratio [increase by 1], duration of ECMO cannulation, time from symptoms to mechanical ventilation and time from mechanical ventilation to ECMO [days]). For continuous variables, we pooled the means from the aggregate data presented in each study as per Wan et al. [15]. A p value of < 0.05 was defined as statistically significant for our analyses. We performed all statistical analyses using R 4.0.2.
Post hoc analysis
We investigated the impact of time of last patient enrolment from Jan 1, 2020 on the duration of ECMO, ICU and hospital lengths of stay using study-level meta-regression. In addition, given the disparity in sample sizes, we conducted an exploratory meta-regression of sample size with mortality rates. As studies might recruit patients over a period of time, we conducted a meta-regression of the mean date of patient enrolment (defined as the midpoint between the date of first and last patient enrolment within each study) and mortality. Finally, we conducted an exploratory subgroup analysis based on the duration of follow-up reported by each study.
Role of the funding source
There was no funding source for this study.
Results
Study selection and characteristics
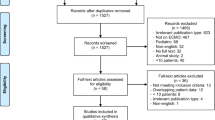
Of 4522 citations, we reviewed 222 full-texts and included 52 studies totalling 18,211 patients receiving ECMO for COVID-19, in the meta-analysis (Fig. 1, Additional file 1: Table S4) [3, 5, 16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,81, 82]. As such, the findings of this review need to be interpreted in context and clinical practice may evolve further.
Conclusions
In conclusion, our review summarising the updated literature on the use of ECMO for COVID-19 demonstrated an increase in mortality in 2021, likely due to a combination of demographic, disease, and intervention factors. It is evident that a one-size fits all protocolised approach to ECMO, used earlier in the pandemic, may not be as applicable as newer variants emerge, clinical patterns vary and management for severe COVID-19 changes. Despite the increase in mortality over time, ECMO still serves an important role as supportive therapy for select patients. Physicians should carefully weigh the potential benefits and harms of ECMO for each patient in the context of resource availability, the individual’s disease course, and local experience and mortality rates in order to decide on ECMO candidacy [7].
Availability of data and materials
All data generated or analysed during this study are included in the published studies and their supplementary information files.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- COVID-19:
-
Coronavirus disease 2019
- ECMO:
-
Extracorporeal membrane oxygenation
- ELSO:
-
Extracorporeal Life Support Organisation
- GRADE:
-
Grading of Recommendations, Assessment, Development and Evaluations
- ICU:
-
Intensive care unit
- IL-6R:
-
Interleukin-6 receptor
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- PROSPERO:
-
International Prospective Register of Systematic Reviews
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- SOFA:
-
Sequential organ failure assessment
- VA:
-
Venoarterial
- VV:
-
Venovenous
References
Ramanathan K, Antognini D, Combes A, Paden M, Zakhary B, Ogino M, et al. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir Med. 2020;8(5):518–26.
Ramanathan K, Shekar K, Ling RR, Barbaro RP, Wong SN, Tan CS, et al. Extracorporeal membrane oxygenation for COVID-19: a systematic review and meta-analysis. Crit Care. 2021;25(1):211.
Barbaro RP, MacLaren G, Boonstra PS, Combes A, Agerstrand C, Annich G, et al. Extracorporeal membrane oxygenation for COVID-19: evolving outcomes from the international Extracorporeal Life Support Organization Registry. Lancet. 2021;398(10307):1230–8.
Barbaro RP, MacLaren G, Boonstra PS, Iwashyna TJ, Slutsky AS, Fan E, et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396(10257):1071–8.
Karagiannidis C, Slutsky AS, Bein T, Windisch W, Weber-Carstens S, Brodie D. Complete countrywide mortality in COVID patients receiving ECMO in Germany throughout the first three waves of the pandemic. Crit Care. 2021;25(1):413.
Mustafa AK, Alexander PJ, Joshi DJ, Tabachnick DR, Cross CA, Pappas PS, et al. Extracorporeal membrane oxygenation for patients with COVID-19 in severe respiratory failure. JAMA Surg. 2020;155(10):990–2.
MacLaren G, Fisher D, Brodie D. Treating the most critically Ill patients with COVID-19: the evolving role of extracorporeal membrane oxygenation. JAMA. 2022;327(1):31–2.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Iorio A, Spencer FA, Falavigna M, Alba C, Lang E, Burnand B, et al. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ. 2015;350: h870.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):39.
Schwarzer G, Chemaitelly H, Abu-Raddad LJ, Rücker G. Seriously misleading results using inverse of Freeman-Tukey double arcsine transformation in meta-analysis of single proportions. Res Synth Methods. 2019;10(3):476–83.
Clopper C, Pearson E. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26(4):404–13.
Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence–inconsistency. J Clin Epidemiol. 2011;64(12):1294–302.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135.
Agerstrand C, Dubois R, Takeda K, Uriel N, Lemaitre P, Fried J, et al. Extracorporeal membrane oxygenation for coronavirus disease 2019: crisis standards of care. Asaio j. 2021;67(3):245–9.
Akhtar W, Olusanya O, Baladia MM, Young H, Shah S. SARS-CoV-2 and ECMO: early results and experience. Indian J Thorac Cardiovasc Surg. 2020;37(1):1–8.
Alnababteh M, Hashmi MD, Vedantam K, Chopra R, Kohli A, Hayat F, et al. Extracorporeal membrane oxygenation for COVID-19 induced hypoxia: Single-center study. Perfusion. 2021;36(6):564–72.
Arachchillage DJ, Rajakaruna I, Scott I, Gaspar M, Odho Z, Banya W, et al. Impact of major bleeding and thrombosis on 180-day survival in patients with severe COVID-19 supported with veno-venous extracorporeal membrane oxygenation in the United Kingdom: a multicentre observational study. Br J Haematol. 2021.
Bergman ZR, Wothe JK, Alwan FS, Lofrano AE, Tointon KM, Doucette M, et al. Risk factors of mortality for patients receiving venovenous extracorporeal membrane oxygenation for COVID-19 Acute respiratory distress syndrome. Surg Infect (Larchmt). 2021;22(10):1086–92.
Bermea RS, Raz Y, Sertic F, Rubin J, Wolf M, Olia S, et al. Increased intracranial hemorrhage amid elevated inflammatory markers in those with COVID-19 supported with extracorporeal membrane oxygenation. Shock. 2021;56(2):206–14.
Bohman JK, Nei SD, Mellon LN, Ashmun RS, Guru PK. Physical therapy and sedation while on extracorporeal membrane oxygenation for COVID-19-associated acute respiratory distress syndrome. J Cardiothorac Vasc Anesth. 2021;S1053–0770(21):00537–41.
Broman LM, Eksborg S, Lo Coco V, De Piero ME, Belohlavek J, Lorusso R. Extracorporeal membrane oxygenation for COVID-19 during first and second waves. Lancet Respir Med. 2021;9(8):e80–1.
Cain MT, Smith NJ, Barash M, Simpson P, Durham LA 3rd, Makker H, et al. Extracorporeal membrane oxygenation with right ventricular assist device for COVID-19 ARDS. J Surg Res. 2021;264:81–9.
Chandel A, Patolia S, Looby M, Bade N, Khangoora V, King CS. Association of D-dimer and fibrinogen with hypercoagulability in COVID-19 requiring extracorporeal membrane oxygenation. J Intensive Care Med. 2021;36(6):689–95.
Cheng W, Ma XD, Su LX, Long Y, Liu DW, Du B, et al. Retrospective study of critically Ill COVID-19 patients with and without extracorporeal membrane oxygenation support in Wuhan, China. Front Med (Lausanne). 2021;8:659793.
Cousin N, Bourel C, Carpentier D, Goutay J, Mugnier A, Labreuche J, et al. SARS-CoV-2 versus influenza-associated acute respiratory distress syndrome requiring veno-venous extracorporeal membrane oxygenation support. Asaio J. 2021;67(2):125–31.
Takeda S. Survey of Critically ill COVID-19 patients in Japan, managed by the Japan ECMOnet for COVID-19 2021 [updated Dec 24.]
Daviet F, Guilloux P, Hraiech S, Tonon D, Velly L, Bourenne J, et al. Impact of obesity on survival in COVID-19 ARDS patients receiving ECMO: results from an ambispective observational cohort. Ann Intensive Care. 2021;11(1):157.
Diaz RA, Graf J, Zambrano JM, Ruiz C, Espinoza JA, Bravo SI, et al. Extracorporeal membrane oxygenation for COVID-19-associated severe acute respiratory distress syndrome in chile: a nationwide incidence and cohort study. Am J Respir Crit Care Med. 2021;204(1):34–43.
Dognon N, Gaudet A, Parmentier-Decrucq E, Normandin S, Vincentelli A, Moussa M, et al. Extracorporeal Membrane Oxygenation for COVID 2019-Acute Respiratory Distress Syndrome: Comparison between First and Second Waves (Stage 2). J Clin Med. 2021;10(21).
Dreier E, Malfertheiner MV, Dienemann T, Fisser C, Foltan M, Geismann F, et al. ECMO in COVID-19-prolonged therapy needed? A retrospective analysis of outcome and prognostic factors. Perfusion. 2021;36(6):582–91.
Durak K, Zayat R, Grottke O, Dreher M, Autschbach R, Marx G, et al. Extracorporeal membrane oxygenation in patients with COVID-19: 1-year experience. J Thorac Dis. 2021;13(10):5911–24.
Falcoz P-E, Monnier A, Puyraveau M, Perrier S, Ludes P-O, Olland A, et al. Extracorporeal membrane oxygenation for critically Ill patients with COVID-19-related acute respiratory distress syndrome: worth the effort? Am J Respir Crit Care Med. 2020;202(3):460–3.
Fang J, Li R, Chen Y, Qin JJ, Hu M, Huang CL, et al. Extracorporeal membrane oxygenation therapy for critically Ill coronavirus disease 2019 patients in Wuhan, China: a retrospective multicenter cohort study. Curr Med Sci. 2021;41(1):1–13.
Huette P, Beyls C, Guilbart M, Coquet A, Berna P, Haye G, et al. Extracorporeal membrane oxygenation for respiratory failure in COVID-19 patients: outcome and time-course of clinical and biological parameters. Can J Anaesth. 2020;67(10):1486–8.
Jacobs JP, Stammers AH, St Louis JD, Hayanga JWA, Firstenberg MS, Mongero LB, et al. Multi-institutional analysis of 200 COVID-19 patients treated with extracorporeal membrane oxygenation: outcomes and trends. Ann Thoracic Surg. 2021.
Jang WS, Kim J, Baek J, Jung H, Jang JS, Park JS, et al. Clinical course of COVID-19 patients treated with ECMO: a multicenter study in Daegu. South Korea Heart Lung. 2021;50(1):21–7.
Jozwiak M, Chiche JD, Charpentier J, Ait Hamou Z, Jaubert P, Benghanem S, et al. Use of venovenous extracorporeal membrane oxygenation in critically-Ill patients with COVID-19. Front Med (Lausanne). 2020;7:614569.
Kon ZN, Smith DE, Chang SH, Goldenberg RM, Angel LF, Carillo JA, et al. Extracorporeal membrane oxygenation support in severe COVID-19. Ann Thorac Surg. 2021;111(2):537–43.
Lai W, Li S, Du Z, Ma X, Lu J, Gao WD, et al. Severe patients with ARDS With COVID-19 treated with extracorporeal membrane oxygenation in China: a retrospective study. Front Med. 2021;8:1837.
Lazzeri C, Bonizzoli M, Batacchi S, Cianchi G, Franci N, Socci F, et al. Persistent right ventricle dilatation in SARS-CoV-2-related acute respiratory distress syndrome on extracorporeal membrane oxygenation support. J Cardiothorac Vasc Anesth. 2021.
Lebreton G, Schmidt M, Ponnaiah M, Folliguet T, Para M, Guihaire J, et al. Extracorporeal membrane oxygenation network organisation and clinical outcomes during the COVID-19 pandemic in Greater Paris, France: a multicentre cohort study. Lancet Respir Med. 2021;9(8):851–62.
Li S, **ong J, Du Z, Lai W, Ma X, Feng Z, et al. Extracorporeal membrane oxygenation (ECMO) for critically ill patients with coronavirus disease 2019 (COVID-19): A retrospective cohort study. J Card Surg. 2021;36(10):3554–60.
Loforte A, Di Mauro M, Pellegrini C, Monterosso C, Pelenghi S, Degani A, et al. Extracorporeal membrane oxygenation for COVID-19 respiratory distress syndrome: an italian society for cardiac surgery report. Asaio j. 2021;67(4):385–91.
Lorusso R, Combes A, Lo Coco V, De Piero ME, Belohlavek J. ECMO for COVID-19 patients in Europe and Israel. Intensive Care Med. 2021;47(3):344–8.
Murthy S, Archambault PM, Atique A, Carrier FM, Cheng MP, Codan C, et al. Characteristics and outcomes of patients with COVID-19 admitted to hospital and intensive care in the first phase of the pandemic in Canada: a national cohort study. CMAJ Open. 2021;9(1):E181–8.
Nguyen NT, Sullivan B, Sagebin F, Hohmann SF, Amin A, Nahmias J. Analysis of COVID-19 patients with acute respiratory distress syndrome managed with extracorporeal membrane oxygenation at US academic centers. Ann Surg. 2021;274(1):40–4.
O’Neil ER, Lin H, Shamshirsaz AA, Naoum EE, Rycus PR, Alexander PM, et al. Pregnant/peripartum women with COVID-19 high survival with ECMO: An ELSO registry analysis. Am J Respir Crit Care Med. 2022;205(2):248–50.
Olivier P-Y, Ottavy G, Hoff J, Auchabie J, Darreau C, Pierrot M. Prolonged time from intubation to cannulation in VV-ECMO for COVID-19: does it really matter? Crit Care. 2021;25(1):385.
Rabie AA, Azzam MH, Al-Fares AA, Abdelbary A, Mufti HN, Hassan IF, et al. Implementation of new ECMO centers during the COVID-19 pandemic: experience and results from the Middle East and India. Intensive Care Med. 2021;47(8):887–95.
Raff LA, Reid TD, Johnson D, Raff EJ, Schneider AB, Charles AG, et al. Comparative outcomes between COVID-19 and influenza patients placed on veno-venous extracorporeal membrane oxygenation for severe ARDS. Am J Surg. 2021;S0002–9610(21):00233–6.
Rajajee V, Fung CM, Seagly KS, Park PK, Raghavendran K, Machado-Aranda DA, et al. One-year functional, cognitive, and psychological outcomes following the use of extracorporeal membrane oxygenation in coronavirus disease 2019: a prospective study. Crit Care Explor. 2021;3(9):e0537.
Rhoades R, Leong R, Kopenitz J, Thoma B, McDermott L, Dovidio J, et al. Coagulopathy monitoring and anticoagulation management in COVID-19 patients on ECMO: advantages of a heparin anti-Xa-based titration strategy. Thromb Res. 2021;203:1–4.
Riera J, Alcántara S, Bonilla C, Fortuna P, Blandino Ortiz A, Vaz A, et al. Risk factors for mortality in patients with COVID-19 needing extracorporeal respiratory support. Eur Respir J. 2022;59(2):2102463.
Roedl K, Kahn A, Jarczak D, Fischer M, Boenisch O, de Heer G, et al. Clinical characteristics, complications and outcomes of patients with severe acute respiratory distress syndrome related to COVID-19 or influenza requiring extracorporeal membrane oxygenation-a retrospective cohort study. J Clin Med. 2021;10(22).
Saeed O, Tatooles AJ, Farooq M, Schwartz G, Pham DT, Mustafa AK, et al. Characteristics and outcomes of patients with COVID-19 supported by extracorporeal membrane oxygenation: a retrospective multicenter study. J Thorac Cardiovasc Surg. 2021;S0022–5223(21):00801–11.
Shah A, Dave S, Galvagno S, George K, Menne AR, Haase DJ, et al. A dedicated veno-venous extracorporeal membrane oxygenation unit during a respiratory pandemic: lessons learned from COVID-19 part II: clinical management. Membranes (Basel). 2021;11(5).
Supady A, DellaVolpe J, Taccone FS, Scharpf D, Ulmer M, Lepper PM, et al. Outcome prediction in patients with severe COVID-19 requiring extracorporeal membrane oxygenation-a retrospective international multicenter study. Membranes (Basel). 2021;11(3).
Suwalski P, Staromłyński J, Brączkowski J, Bartczak M, Mariani S, Drobiński D, et al. Transition from simple V-V to V-A and Hybrid ECMO configurations in COVID-19 ARDS. Membranes. 2021;11(6):434.
Tabatabai A, Ghneim MH, Kaczorowski DJ, Shah A, Dave S, Haase DJ, et al. Mortality risk assessment in COVID-19 venovenous extracorporeal membrane oxygenation. Ann Thorac Surg. 2021;112(6):1983–9.
Ulukan MO, Karakaya A, Yildiz Y, Oztas DM, Kamilcelebi N, O’zdemir S, et al. A single tertiary center outcomes on cannulation strategies and extracorporeal membrane oxygenation in the treatment of respiratory failure during COVID19 infection. Med Bull Haseki. 2021;59:25–30.
Zhang J, Whebell SF, Sanderson B, Retter A, Daly K, Paul R, et al. Phenotypes of severe COVID-19 ARDS receiving extracorporeal membrane oxygenation. Br J Anaesth. 2021;126(3):e130–2.
Corrêa TD, Midega TD, Timenetsky KT, Cordioli RL, Barbas CSV, Silva Júnior M, et al. Clinical characteristics and outcomes of COVID-19 patients admitted to the intensive care unit during the first year of the pandemic in Brazil: a single center retrospective cohort study. Einstein (Sao Paulo). 2021;19:eAO6739-eAO.
Hermann M, Laxar D, Krall C, Hafner C, Herzog O, Kimberger O, et al. Duration of invasive mechanical ventilation prior to extracorporeal membrane oxygenation is not associated with survival in acute respiratory distress syndrome caused by coronavirus disease 2019. Ann Intensive Care. 2022;12(1):6.
Agarwal A, Rochwerg B, Siemieniuk RA, Agoritsas T, Lamontagne F, Askie L, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370: m3379.
Chaudhuri D, Sasaki K, Karkar A, Sharif S, Lewis K, Mammen MJ, et al. Corticosteroids in COVID-19 and non-COVID-19 ARDS: a systematic review and meta-analysis. Intensive Care Med. 2021;47(5):521–37.
Sinha P, Furfaro D, Cummings MJ, Abrams D, Delucchi K, Maddali MV, et al. Latent class analysis reveals COVID-19-related acute respiratory distress syndrome subgroups with differential responses to corticosteroids. Am J Respir Crit Care Med. 2021;204(11):1274–85.
Voicu S, Goury A, Lacoste-Palasset T, Malissin I, Fanet L, Souissi S, et al. Dismal survival in COVID-19 patients requiring ECMO as rescue therapy after corticosteroid failure. J Pers Med. 2021;11(11):1238.
Gangneux J-P, Dannaoui E, Fekkar A, Luyt C-E, Botterel F, De Prost N, et al. Fungal infections in mechanically ventilated patients with COVID-19 during the first wave: the French multicentre MYCOVID study. Lancet Respir Med. 2022;10(2):180–90.
Grasselli G, Scaravilli V, Mangioni D, Scudeller L, Alagna L, Bartoletti M, et al. Hospital-acquired infections in critically Ill patients with COVID-19. Chest. 2021;160(2):454–65.
Poon WH, Ramanathan K, Ling RR, Yang IX, Tan CS, Schmidt M, et al. Prone positioning during venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care. 2021;25(1):292.
Combes A, Peek GJ, Hajage D, Hardy P, Abrams D, Schmidt M, et al. ECMO for severe ARDS: systematic review and individual patient data meta-analysis. Intensive Care Med. 2020;46(11):2048–57.
Gattinoni L, Vasques F, Quintel M. Use of ECMO in ARDS: does the EOLIA trial really help? Crit Care. 2018;22(1):171.
Sidebotham D. Extracorporeal membrane oxygenation–understanding the evidence: CESAR and beyond. J Extra Corpor Technol. 2011;43(1):P23–6.
Ramanathan K, Cove ME, Caleb MG, Teoh KL, Maclaren G. Ethical dilemmas of adult ECMO: emerging conceptual challenges. J Cardiothorac Vasc Anesth. 2015;29(1):229–33.
Barbaro RP, MacLaren G, Swol J, Slutsky AS, Brodie D. COVID-19 ARDS: getting ventilation right—Authors’ reply. Lancet. 2022;399(10319):22–3.
Schmidt M, Bailey M, Sheldrake J, Hodgson C, Aubron C, Rycus PT, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med. 2014;189(11):1374–82.
Hoechter DJ, Becker-Pennrich AS, Geisler BP, Zwissler B, Irlbeck M, Ramanathan K, et al. Letter to the editor regarding Extracorporeal membrane oxygenation for COVID-19: a systematic review and meta-analysis. Crit Care. 2021;25(1):285.
Bertini P, Guarracino F, Falcone M, Nardelli P, Landoni G, Nocci M, et al. ECMO in COVID-19 patients: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2021. https://doi.org/10.1053/j.jcva.2021.11.006.
Fan BE, Wong SW, Sum CLL, Lim GH, Leung BP, Tan CW, et al. Hypercoagulatbility, endotherliopathy, and inflammation approximating 1 year after recvoery: asessing the long-term outcomes in COVID-19 patients. Am J Hematol. 2022. https://doi.org/10.1002/ajh.26575.
Ortoleva J, Dalia AA. Long-term outcomes are important: extracorporeal membrane oxygenation for COVID-19. J Cardiothorac Vasc Anesth. 2021;35(7):2007–8. https://doi.org/10.1053/j.jcva.2021.02.044.
Acknowledgements
The authors would like to acknowledge the following people for their assistance in providing additional data for our analysis: Jordi Riera, Kevin Roedl, Ahmed Rabie, Sachin Shah, Omar Saeed and Florence Daviet.
Funding
There was no funding source for this study.
Author information
Authors and Affiliations
Contributions
Study design was contributed by KR, RRL, KS, DB; Search strategy and screening of articles were contributed by RRL, JJLS, SNW, KR; Risk of bias assessment was contributed by RRL, JJLS, FA, KR; Data collection was contributed by RRL, JJLS, KR; Data analysis and interpretation were contributed by RRL, CY, KR; Tables and figures were contributed by RRL, JJLS; Drafting of manuscript was contributed by RRL, KR; Critical revision of manuscript for intellectually important content was contributed by RRL, KR, FA, SMF, BR, EF, RPB, GM, KR, DB; All authors provided critical conceptual input, interpreted the data analysis, read, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Dr. Ramanathan serves as a co-chair of the Scientific Oversight Committee of the Extracorporeal Life support Organisation (ELSO) and reports honoraria for educational lectures from Baxter. Dr. Fan reports personal fees from ALung Technologies, Aerogen, Baxter, Boehringer-Ingelheim, GE Healthcare, Inspira, and Vasomune outside the submitted work. He is Chair of the ELSO Research Committee and a member of the Executive Committee of the International ECMO Network (ECMONet). Dr. MacLaren serves on the board of directors for ELSO. Dr. Brodie receives research support from ALung Technologies. He has been on the medical advisory boards for Abiomed, Xenios, Medtronic and Cellenkos. He is the President-elect of ELSO and the Chair of the Executive Committee ECMONet. All other authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Supplementary Appendix (Figures S1 to S8, Tables S1 to S8).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ling, R.R., Ramanathan, K., Sim, J.J.L. et al. Evolving outcomes of extracorporeal membrane oxygenation during the first 2 years of the COVID-19 pandemic: a systematic review and meta-analysis. Crit Care 26, 147 (2022). https://doi.org/10.1186/s13054-022-04011-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-022-04011-2