Abstract
Background
Pancreatic cancer is a highly lethal malignancy with poor prognosis. Anillin (ANLN), an actin binding protein, is upregulated and plays an important role in many malignant tumors. However, the precise role of ANLN in pancreatic cancer remains unclear.
Methods
The expression of ANLN and its association with pancreatic cancer patient survival were analyzed using an online database and confirmed by immunohistochemistry. The ANLN protein expression in pancreatic cancer cell lines was detected by Western blot. Cell proliferation, colony formation and transwell assays in vitro and in vivo tumor growth were used to determine the role of ANLN in pancreatic cancer. Gene expression microarray analysis and a series of in vitro assays were used to elucidate the mechanisms of ANLN regulating pancreatic cancer progression.
Results
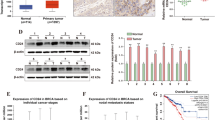
We found that the ANLN expression was significantly upregulated in pancreatic cancer tissues and cell lines. The high expression of ANLN was associated with tumor size, tumor differentiation, TNM stage, lymph node metastasis, distant metastasis and poor prognosis in pancreatic cancer. ANLN downregulation significantly inhibited cell proliferation, colony formation, migration, invasion and tumorigenicity in nude mice. Meanwhile, we found that ANLN knockdown inhibited several cell-cell adhesion related genes, including the gene encoding LIM and SH3 protein 1 (LASP1). LASP1 upregulation partially reversed the tumor-suppressive effect of ANLN downregulation on pancreatic cancer cell progression. Moreover, we found that ANLN downregulation induced the expression of miR-218-5p which inhibited LASP1 expression through binding to its 3’UTR. We also found that ANLN-induced enhancer of zeste homolog 2 (EZH2) upregulation was involved in regulating miR-218-5p/LASP1 signaling axis. EZH2 upregulation or miR-218-5p downregulation partially reversed the tumor-suppressive effect of ANLN downregulation on pancreatic cancer cell progression.
Conclusion
ANLN contributed to pancreatic cancer progression by regulating EZH2/miR-218-5p/LASP1 signaling axis. These findings suggest that ANLN may be a candidate therapeutic target in pancreatic cancer.
Similar content being viewed by others
Background
Pancreatic cancer is a fatal malignancy with a poor prognosis worldwide [1]. Due to occult onset and non-specific symptoms, 80% of patients diagnosed with pancreatic cancer are in advanced stages and have a 5-year survival rate of less than 5% [2, 3]. Despite ongoing advances for the survival rates noted in many cancers, such as colon cancer and breast cancer, the annual mortality rates for patients with pancreatic cancer remain almost equal to the incidence rates [3, 4]. Thus, to improve the health outcomes of pancreatic cancer patients, more intensive efforts should be made to understand the molecular mechanisms underlying pancreatic cancer progression.
Anillin (ANLN), an actin binding protein, first identified in Drosophila, is located on chromosome 7p14.2 and encodes an actin-binding protein that consists of 1125 amino acids and plays an important role in cytokinesis [5,6,7]. In normal tissues, ANLN expression is higher in the placenta, brain and testis, and lower in the lung, heart, liver and spleen [8]. Many recent studies suggests that ANLN is upregulated in numerous cancer types, including cervical cancer, prostate cancer, anaplastic thyroid carcinoma, breast cancer, lung carcinogenesis, bladder urothelial carcinoma, pancreatic cancer and nasopharyngeal carcinoma [9,10,11,12,13,14, All data generated or analyzed during this study are included in this published article and its additional files. Anillin actin binding protein Cell counting kit-8 Enhancer of zeste homolog 2 Gene ontology Immunohistochemistry LIM and SH3 protein 1 Multiplicity of infection Overall survival Quantitative reverse transcription polymerase chain reaction Short hairpin RNA Small interfering RNA Untranslated region Lin QJ, Yang F, ** C, Fu DL. Current status and progress of pancreatic cancer in China. World J Gastroenterol. 2015;21(26):7988–8003. Li XD, Gao PJ, Wang Y, Wang XC. Blood-derived microRNAs for pancreatic cancer diagnosis: a narrative review and meta-analysis. Front Physiol. 2018;9:685. Rossi ML, Rehman AA, Gondi CS. Therapeutic options for the management of pancreatic cancer. World J Gastroenterol. 2014;20(32):11142–59. Adamska A, Falasca M. ATP-binding cassette transporters in progression and clinical outcome of pancreatic cancer: what is the way forward? World J Gastroenterol. 2018;24(29):3222–38. Field CM, Alberts BM. Anillin, a contractile ring protein that cycles from the nucleus to the cell cortex. J Cell Biol. 1995;131(1):165–78. Hickson GR, O'Farrell PH. Anillin: a pivotal organizer of the cytokinetic machinery. Biochem Soc Trans. 2008;36:439–41. Piekny AJ, Maddox AS. The myriad roles of Anillin during cytokinesis. Semin Cell Dev Biol. 2010;21(9):881–91. Oegema K, Savoian MS, Mitchison TJ, Field CM. Functional analysis of a human homologue of the Drosophila actin binding protein anillin suggests a role in cytokinesis. J Cell Biol. 2000;150(3):539–52. Long XY, Zhou W, Wang YX, Liu SQ. Prognostic significance of ANLN in lung adenocarcinoma. Oncol Lett. 2018;16(2):1835–40. Olakowski M, Tyszkiewicz T, Jarzab M, Król R, Oczko-Wojciechowska M, Kowalska M, Kowal M, Gala GM, Kajor M, Lange D, et al. NBL1 and anillin (ANLN) genes over-expression in pancreatic carcinoma. Folia Histochem Cytobiol. 2009;47(2):249–55. Sadi AM, Wang DY, Youngson BJ, Miller N, Boerner S, Done SJ, Leong WL. Clinical relevance of DNA microarray analyses using archival formalin-fixed paraffin-embedded breast cancer specimens. BMC Cancer. 2011;11:1–13. Tamura K, Furihata M, Tsunoda T, Ashida S, Takata R, Obara W, Yoshioka H, Daigo Y, Nasu Y, Kumon H, et al. Molecular features of hormone-refractory prostate cancer cells by genome-wide gene expression profiles. Cancer Res. 2007;67(11):5117–25. Wang SM, Mo YX, Midorikawa K, Zhang Z, Huang GW, Ma N, Zhao WL, Hiraku Y, Oikawa S, Murata M. The potent tumor suppressor miR-497 inhibits cancer phenotypes in nasopharyngeal carcinoma by targeting ANLN and HSPA4L. Oncotarget. 2015;6(34):35893–907. Weinberger P, Ponny SR, Xu HY, Bai S, Smallridge R, Copland J, Sharma A. Cell cycle M-phase genes are highly upregulated in anaplastic thyroid carcinoma. Thyroid. 2017;27(2):236–52. **a LL, Su XL, Shen JZ, Meng Q, Yan JQ, Zhang CH, Chen Y, Wang H, Xu MJ. ANLN functions as a key candidate gene in cervical cancer as determined by integrated bioinformatic analysis. Cancer Manag Res. 2018;10:663–70. Zeng SX, Yu XW, Ma C, Song RX, Zhang ZS, Zi X, Chen X, Wang Y, Yu YW, Zhao JJ, et al. Transcriptome sequencing identifies ANLN as a promising prognostic biomarker in bladder urothelial carcinoma. Sci Rep. 2017;7(1):3151. Suzuki C, Daigo Y, Ishikawa N, Kato T, Hayama S, Ito T, Tsuchiya E, Nakamura Y. ANLN plays a critical role in human lung carcinogenesis through the activation of RHOA and by involvement in the phosphoinositide 3-kinase/AKT pathway. Cancer Res. 2005;65(24):11314–25. Idichi T, Seki N, Kurahara H, Yonemori K, Osako Y, Arai T, Okato A, Kita Y, Arigami T, Mataki Y, et al. Regulation of actin-binding protein ANLN by antitumor miR-217 inhibits cancer cell aggressiveness in pancreatic ductal adenocarcinoma. Oncotarget. 2017;8(32):53180–93. Tomasetto C, Régnier C, Moog-Lutz C, Mattei MG, Chenard MP, Lidereau R, Basset P, Rio MC. Identifcation of four novel human genes amplifed and overexpressed in breast carcinoma and localized to the q11-q21.3 region of chromosome 17. Genomics. 1995;28:367–76. Zhou R, Shao ZY, Liu J, Zhan WQ, Gao QZ, Pan ZH, Wu L, Xu LJ, Ding YQ, Zhao L. COPS5 and LASP1 synergistically interact to downregulate 14-3-3σ expression and promote colorectal cancer progression via activating PI3K/AKT pathway. Int J Cancer. 2018;142(9):1853–64. Endres M, Kneitz S, Orth MF, Perera RK, Zernecke A, Butt E. Regulation of matrix metalloproteinases (MMPs) expression and secretion in MDA-MB-231 breast cancer cells by LIM and SH3 protein 1 (LASP1). Oncotarget. 2016;7:64244–59. Zhong CH, Chen YT, Tao B, Peng LL, Peng TM, Yang XB, **a XG, Chen LG. LIM and SH3 protein 1 regulates cell growth and chemosensitivity of human glioblastoma via the PI3K/AKT pathway. BMC Cancer. 2018;18(1):722. Zhao L, Wang H, Liu C, Liu YW, Wang XY, Wang S, Sun XG, Li JM, Deng YJ, Jiang Y, Ding YQ. Promotion of colorectal cancer growth and metastasis by the LIM and SH3 domain protein 1. Gut. 2010;59:1226–35. Zhao TS, Ren H, Li J, Chen J, Zhang H, **n W, Sun Y, Sun L, Yang YW, Sun JW, et al. LASP1 is a HIF1α target gene critical for metastasis of pancreatic cancer. Cancer Res. 2015;75(1):111–9. Vorvis C, Koutsioumpa M, Iliopoulos D. Developments in miRNA gene signaling pathways in pancreatic cancer. Future Oncol. 2016;12(9):1135–50. Liu M, Yin K, Guo X, Feng H, Yuan M, Liu Y, Zhang J, Guo B, Wang C, Zhou G, et al. Diphthamide biosynthesis 1 is a novel oncogene in colorectal Cancer cells and is regulated by MiR-218-5p. Cell Physiol Biochem. 2017;44(2):505–14. Xu YS, He Q, Lu YY, Tao FX, Zhao L, Ou RY. MicroRNA-218-5p inhibits cell growth and metastasis in cervical cancer via LYN/NF-κB signaling pathway. Cancer Cell Int. 2018;18:198. Deng M, Zeng C, Lu XH, He XS, Zhang RX, Qiu QW, Zheng GP, Jia XT, Liu H, He ZM. miR-218 suppresses gastric cancer cell cycle progression through the CDK6/cyclin D1/E2F1 axis in a feedback loop. Cancer Lett. 2017;403:175–85. Li BS, Liu H, Yang WL. Reduced miRNA-218 expression in pancreatic cancer patients as a predictor of poor prognosis. Genet Mol Res. 2015;14(4):16372–8. Liu Z, Xu Y, Long J, Guo K, Ge C, Du R. microRNA-218 suppresses the proliferation, invasion and promotes apoptosis of pancreatic cancer cells by targeting HMGB1. Chin J Cancer Res. 2015;27(3):247–57. Wang LL, Wang L, Wang XY, Shang D, Yin SJ, Sun LL, Ji HB. MicroRNA-218 inhibits the proliferation, migration, and invasion and promotes apoptosis of gastric cancer cells by targeting LASP1. Tumour Biol. 2016;37(11):15241–52. Nishikawa R, Goto Y, Sakamoto S, Chiyomaru T, Enokida H, Kojima S, Kinoshita T, Yamamoto N, Nakagawa M, Naya Y, et al. Tumor-suppressive microRNA-218 inhibits cancer cell migration and invasion via targeting of LASP1 in prostate cancer. Cancer Sci. 2014;105(7):802–11. Shahabipour F, Caraglia M, Majeed M, Derosa G, Maffioli P, Sahebkar A. Naturally occurring anti-cancer agents targeting EZH2. Cancer Lett. 2017;400:325–35. Yamagishi M, Uchimaru K. Targeting EZH2 in cancer therapy. Curr Opin Oncol. 2017;29(5):375–81. Cao Q, Mani RS, Ateeq B, Dhanasekaran SM, Asangani IA, Prensner JR, Kim JH, Brenner JC, **g X, Cao X, et al. Coordinated regulation of polycomb group complexes through microRNAs in cancer. Cancer Cell. 2011;20:187–99. Ma J, Zhang J, Weng YC, Wang JC. EZH2-mediated microRNA-139-5p regulates epithelial-mesenchymal transition and lymph node metastasis of pancreatic Cancer. Mol Cells. 2018;41(9):868–80. Li CH, To KF, Tong JH, **ao Z, **a T, Lai PB, Chow SC, Zhu YX, Chan SL, Marquez VE, et al. Enhancer of zeste homolog 2 silences microRNA-218 in human pancreatic ductaladenocarcinoma cells by inducing formation of heterochromatin. Gastroenterology. 2013;144(5):1086–97. Kang W, Tong JH, Chan AW, Lee TL, Lung RW, Leung PP, So KK, Wu K, Fan D, Yu J, et al. Yes-associated protein 1 exhibits oncogenic property in gastric cancer and its nuclear accumulation associates with poor prognosis. Clin Cancer Res. 2011;17(8):2130–9. Shin G, Kang TW, Yang S, Baek SJ, Jeong YS, Kim SY. GENT: gene expression database of normal and tumor tissues. Cancer Inform. 2011;10:149–57. Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–4. Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. Brady-Kalnay SM. Molecular mechanisms of cancer cell-cell interactions: cell-cell adhesion-dependent signaling in the tumor microenvironment. Cell Adhes Migr. 2012;6(4):344–5. Hale JS, Li M, Lathia JD. The malignant social network: cell-cell adhesion and communication in cancer stem cells. Cell Adhes Migr. 2012;6(4):346–55. Taniuchi K, Furihata M, Iwasaki S, Tanaka K, Shimizu T, Saito M, Saibara T. RUVBL1 directly binds actin filaments and induces formation of cell protrusions to promote pancreatic cancer cell invasion. Int J Oncol. 2014;44(6):1945–54. Lian Y, **ong F, Yang LT, Bo H, Gong ZJ, Wang YM, Wei F, Tang YY, Li XY, Liao QJ, et al. Long noncoding RNA AFAP1-AS1 acts AS a competing endogenous RNA of miR-423-5p to facilitate nasopharyngeal carcinoma metastasis through regulating the rho/Rac pathway. J Exp Clin Cancer Res. 2018;37(1):253. Zhang HR, Lai SY, Huang LJ, Zhang ZF, Liu J, Zheng SR, Ding K, Bai X, Zhou JY. Myosin 1b promotes cell proliferation, migration, and invasion in cervical cancer. Gynecol Oncol. 2018;149(1):188–97. Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2014;9:287–314. Vaughan L, Tan CT, Chapman A, Nonaka D, Mack NA, Smith D, Booton R, Hurlstone AF, Malliri A. HUWE1 ubiquitylates and degrades the RAC activator TIAM1 promoting cell-cell adhesion disassembly, migration, and invasion. Cell Rep. 2015;10(1):88–102. Naor D, Nedvetzki S, Golan I, Melnik L, Faitelson Y. CD44 in cancer. Crit Rev Clin Lab Sci. 2002;39(6):527–79. Ozen M, Karatas OF, Gulluoglu S, Bayrak OF, Sevli S, Guzel E, Ekici ID, Caskurlu T, Solak M, Creighton CJ, et al. Overexpression of miR-145-5p inhibits proliferation of prostate cancer cells and reduces SOX2 expression. Cancer Investig. 2015;33(6):251–8. Wang J, Wang B, Ren HQ, Chen W. miR-9-5p inhibits pancreatic cancer cell proliferation, invasion and glutamine metabolism by targeting GOT1. Biochem Biophys Res Commun. 2019;509(1):241–8. Fang M, Du HC, Han B, **a GY, Shi XL, Zhang F, Fu QQ, Zhang T. Hypoxia-inducible microRNA-218 inhibits trophoblast invasion by targeting LASP1: implications for preeclampsia development. Int J Biochem Cell Biol. 2017;87:95–103. Wang BR, Liu Y, Luo F, Xu Y, Qin Y, Lu XL, Xu WC, Shi L, Liu QZ, **ang QY. Epigenetic silencing of microRNA-218 via EZH2-mediated H3K27 trimethylation is involved in malignant transformation of HBE cells induced by cigarette smoke extract. Arch Toxicol. 2016;90(2):449–61. Zhang XP, Liu Y, Fan CF, Wang L, Li AL, Zhou HJ, Cai L, Miao Y, Li QC, Qiu XS, et al. Lasp1 promotes malignant phenotype of non-small-cell lung cancer via inducing phosphorylation of FAK-AKT pathway. Oncotarget. 2017;8(43):75102–13. Gao QZ, Tang LH, Wu L, Li KT, Wang H, Li WD, Wu J, Li MY, Wang S, Zhao L. LASP1 promotes nasopharyngeal carcinoma progression through negatively regulation of the tumor suppressor PTEN. Cell Death Dis. 2018;9(3):393. Ebrahimi S, Hosseini M, Shahidsales S, Maftouh M, Ferns GA, Ghayour-Mobarhan M, Hassanian SM, Avan A. Targeting the Akt/PI3K signaling pathway as a potential therapeutic strategy for the treatment of pancreatic Cancer. Curr Med Chem. 2017;24(13):1321–31. Not applicable. This study was supported by the National Natural Science Foundation of China (no. 81472288 and no. 81472775). ABW, HSD, WL and PB designed the experiments; ABW and HSD performed the experiments and analyzed the data; YG, CCZ, JJS, YDL and YJ collected and analyzed the clinical samples; ABW and HSD drafted the manuscript; WL and PB reviewed the manuscript. All authors have read and approved the final manuscript. This study was approved by the Ethical Committee of Army Medical University, China. All patients provided written informed consent to participate in the study. The authors declare that they agree to submit the article for publication. The authors declare that they have no competing interests. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Table S1. siRNAs, shRNA and primers. (DOCX 20 kb) Table S2. GO terms representing biological process. (DOCX 17 kb) Table S3. GO terms representing molecular function. (DOCX 17 kb) Table S4. GO terms representing cellular compartment. (DOCX 19 kb) Figure S1. ANLN knockdown inhibited BxPC-3 cell proliferation by mediating LASP1. (A) Based on the GENT database, LASP1, RAB11B, RUVBL1 and MYO1B gene expression were analyzed in the pancreatic cancer tissues and normal pancreatic tissues. (B) There were significant Pearson correlations of ANLN with LASP1 and ANLN with RUVBL1 in the pancreatic cancer tissues and the normal pancreatic tissues. (C) CCK-8 assay showed that LASP1 restoration partially reversed the effects of ANLN knockdown on pancreatic cancer cell proliferation, while RUVBL1 restoration did not reverse the effect of ANLN downregulation on pancreatic cancer cell proliferation. **P < 0.01. (JPG 1806 kb) Figure S2. MiR-218-5p upregulation significantly repressed the expression of LASP1 mRNA in BxPC-3. (A) The heat map of the differentially expressed miRNA precursors with a fold change of greater than 2 or less than − 2 in ANLN RNAi relative to NC. (B) The selected candidate miRNAs (miR-145-5p, miR-218-5p and miR-9-5p) were confirmed by qRT-PCR after ANLN RNAi transfection in BxPC-3 cells. (C) The expression levels of miR-145-5p, miR-218-5p and miR-9-5p were detected by qRT-PCR in BxPC-3 cells transfected with the miR-145-5p mimic (miR-145-5p), miR-218-5p mimic (miR-218-5p), miR-9-5p mimic (miR-9-5p) or mimic control (con). U6 was used as a loading control. (D) The expression levels of LASP1 mRNA in BxPC-3 cells transfected with the miR-145-5p mimic (miR-145-5p), miR-218-5p mimic (miR-218-5p), miR-9-5p mimic (miR-9-5p) or mimic control (con) were analyzed by qRT-PCR. **P < 0.01. (JPG 1590 kb) Figure S3. ANLN depletion significantly repressed the expression of EZH2 mRNA and protein in BxPC-3 and SW1990 cells. (A) The heat map of the selected genes. (B and C) The expression of EZH2 mRNA and protein was determined by qRT-PCR and Western blot in BxPC-3 and SW1990 cells transfected with the ANLN siRNA (ANLN RNAi) or the scramble control (NC). (JPG 512 kb) Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated. Wang, A., Dai, H., Gong, Y. et al. ANLN-induced EZH2 upregulation promotes pancreatic cancer progression by mediating miR-218-5p/LASP1 signaling axis.
J Exp Clin Cancer Res 38, 347 (2019). https://doi.org/10.1186/s13046-019-1340-7 Received: Accepted: Published: DOI: https://doi.org/10.1186/s13046-019-1340-7Availability of data and materials
Abbreviations
References
Acknowledgements
Funding
Author information
Authors and Affiliations
Contributions
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Consent for publication
Competing interests
Additional information
Publisher’s Note
Additional files
Additional file 1:
Additional file 2:
Additional file 3:
Additional file 4:
Additional file 5:
Additional file 6:
Additional file 7:
Rights and permissions
About this article
Cite this article
Keywords