Abstract
Background
Lycorine has been revealed to inhibit the development of many kinds of malignant tumors, including glioblastoma multiforme (GBM). Although compelling evidences demonstrated Lycorine’s inhibition on cancers through some peripheral mechanism, in-depth mechanism studies of Lycotine’s anti-GBM effects still call for further exploration. Epidermal Growth Factor Receptor (EGFR) gene amplification and mutations are the most common oncogenic events in GBM. Targeting EGFR by small molecular inhibitors is a rational strategy for GBM treatment.
Methods
The molecular docking modeling and in vitro EGFR kinase activity system were employed to identify the potential inhibitory effects of Lycorine on EGFR. And the Biacore assay was used to confirm the direct binding status between Lycorine and the intracellular EGFR (696–1022) domain. In vitro assays were conducted to test the suppression of Lycorine on the biological behavior of GBM cells. By RNA interference, EGFR expression was reduced then cells underwent proliferation assay to investigate whether Lycorine’s inhibition on GBM cells was EGFR-dependent or not. RT-PCR and western blotting analysis were carried out to investigate the underlined molecular mechanism that Lycorine exerted on EGFR itself and EGFR signaling pathway. Three different xenograft models (an U251-luc intracranially orthotopic transplantation model, an EGFR stably knockdown U251 subcutaneous xenograft model and a patient-derived xenograft model) were performed to verify Lycorine’s therapeutic potential on GBM in vivo.
Results
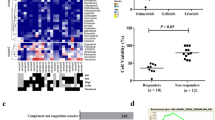
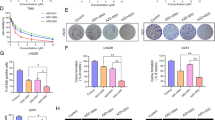
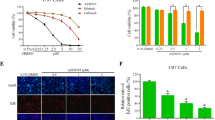
We identified a novel small natural molecule Lycorine binding to the intracellular EGFR (696–1022) domain as an inhibitor of EGFR. Lycorine decreased GBM cell proliferation, migration and colony formation by inducing cell apoptosis in an EGFR-mediated manner. Furthermore, Lycorine inhibited the xenograft tumor growths in three animal models in vivo. Besides, Lycorine impaired the phosphorylation of EGFR, AKT, which were mechanistically associated with expression alteration of a series of cell survival and death regulators and metastasis-related MMP9 protein.
Conclusions
Our findings identify Lycorine directly interacts with EGFR and inhibits EGFR activation. The most significant result is that Lycorine displays satisfactory therapeutic effect in our patient-derived GBM tumor xenograft, thus supporting the conclusion that Lycorine may be considered as a promising candidate in clinical therapy for GBM.
Similar content being viewed by others
Background
Gliomas are the most common brain tumor in adults, accounting for about 70% of primary neoplasms of the central nervous system (CNS). High-grade gliomas, especially the glioblastoma multiforme (GBM), is the most common and progressive type during all intracranial cancers [1]. About 90% of GBMs are classified as primary and associated with dismal prognosis that typically appears suddenly in patients. On one hand, such lesions affect mainly the elderly (mean age 62 years), have rapid evolution (less than 3 months) and no clinical or histopathological evidence of precursor lesions [2]. On the other hand, secondary GBMs affect younger individuals (average age 45 years) and progress slowly from a lower degree of diffuse astrocytoma. Current therapeutic strategies for GBM include surgical resection, followed by radiotherapy and chemotherapy [3,4,5]. Despite such aggressive multimodal therapy, the median survival of GBM is still poor [6]. The high mortality rate results from the universal resurgence of tumors post-treatment, which occurs due to infiltrating tumor cells that escape initial surgery and exhibit profound resistance to irradiation and current chemotherapy treatments [7]. With the increasing number of cancer-related mortality, identification of novel tractable targets for improved therapeutics and development for novel drugs that can radically cure GBM are desperately needed.
Genomic and proteomic analyses have identified a number of key oncogenic drivers of GBM tumorigenesis and therapeutic resistance, including receptor tyrosine kinases (RTKs) [8]. In particular, genomic alteration of the epidermal growth factor receptor (EGFR) is present in approximately half of all GBMs [9, 10]. EGFR plays an important role in various tumors including GBM. It is the most frequently amplified gene in GBM, while its expression in normal brain tissue is either undetectable or extremely low [11]. The most common genetic aberration associated with malignant glioma is amplification of EGFR, with a frequency of about 50%. Amplifications and rearrangements of EGFR are highly indicative of high-grade gliomas, with a worse prognosis than estimated from just histopathologic grading [12]. EGFR activation leads to autophosphorylation of several key tyrosine residues triggering several intracellular downstream signaling pathways including the Ras/Raf/MEK/ERK pathway, the PLCγ -PKC pathway and the PI3K/AKT pathway, resulting in cell proliferation, motility and survival [13]. Within such a large proportion of EGFR genomic alterations, approximately 20–40% of them harbor EGFR variant III (EGFRvIII) mutant, which contains a deletion of exons 2–7 in the extracellular ligand-binding domain [14, 15]. EGFRvIII induces the receptor tyrosine kinase activation in both a cell autonomous and nonautonomous manner, thus results in a ligand-independent mutant and shows constitutive activation in the absence of ligand to activate the tumor-promoting signaling pathways [16].
The fact that EGFR functions one of the most vital factors to promote gliomas has attracted many investigations of EGFR inhibitors, aiming to promote apoptosis of cancer cells, or to increase tumor sensitivity to possible adjuvant therapies. However, the successful application of EGFR-targeted therapy for the treatment of GBM has proven to be very challenging. Many GBM patients do not respond to these therapies and eventually show drug resistance and disease progression [16]. To screen and develop novel inhibitors that target both wild type EGFR and EGFRvIII to impair GBM malignant tumor cell biology could be therapeutically beneficial either as single agents or in combination with other chemotherapy agents in gliomas therapy.
Lycorine is a pyrrolo[de]phenanthridine ring-type alkaloid extracted from Amaryllidaceae genera and possesses various biological effects including anti-tumor [17], antiviral [18], antimalarial [19], and antiinflammation [20]. Several studies have shown that Lycorine exhibits selective cytotoxicity effects on leukemia, cervical cancer, multiple myeloma, prostate cancer, hepatocellular carcinoma, bladder cancer and breast cancer [21,22,4c and d). And these result also differ two situations when treating cells with long time Lycorine (Fig. 4c) or short time Lycorine (Fig. 4d). If cells were treated with Lycorine initially and then stimulated with EGF, Lycorine could enter the cytoplasm, bind with intracellular EGFR (696–1022) domain and occupy the ATP binding pocket of intracellular EGFR, which might hinder EGFR autophosphorylation, because Lycorine might block the essential binding process of ATP and EGFR for EGFR’s auto-activated phosphorylation. Thus even if cells were stimulated by EGF, the level of p-EGFR was still too low to be detected under Lycorine pretreated groups (Fig. 5b and c). Anyhow, our findings prove that Lycorine inhibits EGF activation of EGFR kinase activity. We may also infer that the extracellular EGF be no inclined to have any relationship with intracellular Lycorine. However, our present study indeed finds Lycorine reduces the mRNA level of EGF and EGFR in vivo (Fig. 6c) and down-regulates both total EGFR and p-EGFR in vitro (Fig. 4c). The intrinsic regulation mechanism between Lycorine and EGF/EGFR is still cryptic. Why Lycorine can affect the transcription of EGF? How Lycorine can reduce the protein expression of EGFR? Whether Lycorine can regulate EGFR’s endocytosis, degradation, cycling, and nuclear translocation and so on? All these detailed mechanisms between Lycorine and EGF/EGFR need to be further explored.
Conclusions
To sum up, our findings confirm Lycorine inhibits GBM growth through EGFR suppression in terms of the way that Lycorine treatment reduces EGFR expression level and inactive EGFR downstream signaling pathway through direct binding to EGFR. Our research provides a proof-of-principle that targeting the alternatively amplified and mutated EGFR by Lycorine could be used to substitute existing EGFR inhibitors and hinder GBM tumor growth.
Abbreviations
- BBB:
-
blood-brain barrier
- CNS:
-
Central nervous system
- EGFR:
-
Epidermal growth factor receptor
- EGFRvIII:
-
EGFR variant III mutant
- GBM:
-
Glioblastoma multiforme
- IC50 :
-
Half maximal inhibitory concentration
- MMP9:
-
Matrix metalloproteinase 9
- PDB:
-
Protein Data Bank
- RTKs:
-
Receptor tyrosine kinases
- RT-PCR:
-
reverse transcription-polymerase chain reaction
- SPR:
-
surface plasmon resonance
References
Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, Wolinsky Y, Kruchko C, Barnholtz-Sloan J: CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro-Oncology 2014, 16 Suppl 4:iv1–63.
Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507.
Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64(6):479–89.
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96.
DeWitt JC, Mock A, Louis DN. The 2016 WHO classification of central nervous system tumors: what neurologists need to know. Curr Opin Neurol. 2017;
Delgado-Lopez PD, Corrales-Garcia EM. Survival in glioblastoma: a review on the impact of treatment modalities. Clin Transl Oncol. 2016;18(11):1062–71.
Claes A, Idema AJ, Wesseling P. Diffuse glioma growth: a guerilla war. Acta Neuropathol. 2007;114(5):443–58.
Beroukhim R, Getz G, Nghiemphu L, Barretina J, Hsueh T, Linhart D, Vivanco I, Lee JC, Huang JH, Alexander S, et al. Assessing the significance of chromosomal aberrations in cancer: methodology and application to glioma. Proc Natl Acad Sci U S A. 2007;104(50):20007–12.
Hurtt MR, Moossy J, Donovan-Peluso M, Locker J. Amplification of epidermal growth factor receptor gene in gliomas: histopathology and prognosis. J Neuropathol Exp Neurol. 1992;51(1):84–90.
Jaros E, Perry RH, Adam L, Kelly PJ, Crawford PJ, Kalbag RM, Mendelow AD, Sengupta RP, Pearson AD. Prognostic implications of p53 protein, epidermal growth factor receptor, and Ki-67 labelling in brain tumours. Br J Cancer. 1992;66(2):373–85.
Gollapalli K, Ghantasala S, Atak A, Rapole S, Moiyadi A, Epari S, Srivastava S. Tissue proteome analysis of different grades of human gliomas provides major cues for glioma pathogenesis. OMICS. 2017;21(5):275–84.
Pelloski CE, Ballman KV, Furth AF, Zhang L, Lin E, Sulman EP, Bhat K, McDonald JM, Yung WK, Colman H, et al. Epidermal growth factor receptor variant III status defines clinically distinct subtypes of glioblastoma. J Clin Oncol. 2007;25(16):2288–94.
Zandi R, Larsen AB, Andersen P, Stockhausen MT, Poulsen HS. Mechanisms for oncogenic activation of the epidermal growth factor receptor. Cell Signal. 2007;19(10):2013–23.
Biernat W, Huang H, Yokoo H, Kleihues P, Ohgaki H. Predominant expression of mutant EGFR (EGFRvIII) is rare in primary glioblastomas. Brain Pathol. 2004;14(2):131–6.
Fan QW, Cheng CK, Gustafson WC, Charron E, Zipper P, Wong RA, Chen J, Lau J, Knobbe-Thomsen C, Weller M, et al. EGFR phosphorylates tumor-derived EGFRvIII driving STAT3/5 and progression in glioblastoma. Cancer Cell. 2013;24(4):438–49.
Ohno M, Natsume A, Ichiro Iwami K, Iwamizu H, Noritake K, Ito D, Toi Y, Ito M, Motomura K, Yoshida J, et al. Retrovirally engineered T-cell-based immunotherapy targeting type III variant epidermal growth factor receptor, a glioma-associated antigen. Cancer Sci. 2010;101(12):2518–24.
Nair JJ, van Staden J. Cytotoxicity studies of lycorine alkaloids of the Amaryllidaceae. Nat Prod Commun. 2014;9(8):1193–210.
Hwang YC, Chu JJ, Yang PL, Chen W, Yates MV. Rapid identification of inhibitors that interfere with poliovirus replication using a cell-based assay. Antivir Res. 2008;77(3):232–6.
Sener B, Orhan I, Satayavivad J. Antimalarial activity screening of some alkaloids and the plant extracts from Amaryllidaceae. Phytother Res. 2003;17(10):1220–3.
Mikami M, Kitahara M, Kitano M, Ariki Y, Mimaki Y, Sashida Y, Yamazaki M, Yui S. Suppressive activity of lycoricidinol (narciclasine) against cytotoxicity of neutrophil-derived calprotectin, and its suppressive effect on rat adjuvant arthritis model. Biol Pharm Bull. 1999;22(7):674–8.
Liu J, Hu WX, He LF, Ye M, Li Y. Effects of lycorine on HL-60 cells via arresting cell cycle and inducing apoptosis. FEBS Lett. 2004;578(3):245–50.
Lamoral-Theys D, Andolfi A, Van Goietsenoven G, Cimmino A, Le Calve B, Wauthoz N, Megalizzi V, Gras T, Bruyere C, Dubois J, et al. Lycorine, the main phenanthridine Amaryllidaceae alkaloid, exhibits significant antitumor activity in cancer cells that display resistance to proapoptotic stimuli: an investigation of structure-activity relationship and mechanistic insight. J Med Chem. 2009;52(20):6244–56.
Hu M, Peng S, He Y, Qin M, Cong X, **ng Y, Liu M, Yi Z. Lycorine is a novel inhibitor of the growth and metastasis of hormone-refractory prostate cancer. Oncotarget. 2015;6(17):15348–61.
Ying X, Huang A, **ng Y, Lan L, Yi Z, He P. Lycorine inhibits breast cancer growth and metastasis via inducing apoptosis and blocking Src/FAK-involved pathway. Sci China Life Sci. 2017;60(4):417–28.
Henry S, Kidner R, Reisenauer MR, Magedov IV, Kiss R, Mathieu V, Lefranc F, Dasari R, Evidente A, Yu X, et al. 5,10b-Ethanophenanthridine amaryllidaceae alkaloids inspire the discovery of novel bicyclic ring systems with activity against drug resistant cancer cells. Eur J Med Chem. 2016;120:313–28.
Dasari R, Banuls LM, Masi M, Pelly SC, Mathieu V, Green IR, van Otterlo WA, Evidente A, Kiss R, Kornienko A. C1,C2-ether derivatives of the Amaryllidaceae alkaloid lycorine: retention of activity of highly lipophilic analogues against cancer cells. Bioorg Med Chem Lett. 2014;24(3):923–7.
Evdokimov NM, Lamoral-Theys D, Mathieu V, Andolfi A, Frolova LV, Pelly SC, van Otterlo WA, Magedov IV, Kiss R, Evidente A, et al. In search of a cytostatic agent derived from the alkaloid lycorine: synthesis and growth inhibitory properties of lycorine derivatives. Bioorg Med Chem. 2011;19(23):7252–61.
Van Goietsenoven G, Andolfi A, Lallemand B, Cimmino A, Lamoral-Theys D, Gras T, Abou-Donia A, Dubois J, Lefranc F, Mathieu V, et al. Amaryllidaceae alkaloids belonging to different structural subgroups display activity against apoptosis-resistant cancer cells. J Nat Prod. 2010;73(7):1223–7.
Li Y, Liu J, Tang LJ, Shi YW, Ren W, Hu WX. Apoptosis induced by lycorine in KM3 cells is associated with the G0/G1 cell cycle arrest. Oncol Rep. 2007;17(2):377–84.
Li L, Dai HJ, Ye M, Wang SL, **ao XJ, Zheng J, Chen HY, Luo YH, Liu J. Lycorine induces cell-cycle arrest in the G0/G1 phase in K562 cells via HDAC inhibition. Cancer Cell Int. 2012;12(1):49.
Yu H, Qiu Y, Pang X, Li J, Wu S, Yin S, Han L, Zhang Y, ** C, Gao X, et al. Lycorine promotes autophagy and apoptosis via TCRP1/Akt/mTOR Axis inactivation in human hepatocellular carcinoma. Mol Cancer Ther. 2017;16(12):2711–23.
Wang C, Wang Q, Li X, ** Z, Xu P, Xu N, Xu A, Xu Y, Zheng S, Zheng J, et al. Lycorine induces apoptosis of bladder cancer T24 cells by inhibiting phospho-Akt and activating the intrinsic apoptotic cascade. Biochem Biophys Res Commun. 2017;483(1):197–202.
Garreau de Loubresse N, Prokhorova I, Holtkamp W, Rodnina MV, Yusupova G, Yusupov M. Structural basis for the inhibition of the eukaryotic ribosome. Nature. 2014;513(7519):517–22.
Shen J, Zhang S, Li Y, Zhang W, Chen J, Zhang M, Wang T, Jiang L, Zou X, Wong J, et al. p14(ARF) inhibits the functions of adenovirus E1A oncoprotein. The Biochemical journal. 2011;434(2):275–85.
Shen J, Sheng X, Chang Z, Wu Q, Wang S, Xuan Z, Li D, Wu Y, Shang Y, Kong X, et al. Iron metabolism regulates p53 signaling through direct heme-p53 interaction and modulation of p53 localization, stability, and function. Cell Rep. 2014;7(1):180–93.
Sun L, Yu S, Xu H, Zheng Y, Lin J, Wu M, Wang J, Wang A, Lan Q, Furnari F, et al. FHL2 interacts with EGFR to promote glioblastoma growth. Oncogene. 2018;37(10):1386–98.
Li C, Hu J, Li W, Song G, Shen J. Combined bortezomib-based chemotherapy and p53 gene therapy using hollow mesoporous silica nanospheres for p53 mutant non-small cell lung cancer treatment. Biomaterials science. 2016;5(1):77–88.
Shen J, Song G, An M, Li X, Wu N, Ruan K, Hu J, Hu R. The use of hollow mesoporous silica nanospheres to encapsulate bortezomib and improve efficacy for non-small cell lung cancer therapy. Biomaterials. 2014;35(1):316–26.
Shen J, Ma H, Zhang T, Liu H, Yu L, Li G, Li H, Hu M. Magnolol inhibits the growth of non-small cell lung Cancer via inhibiting microtubule polymerization. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2017;42(5):1789–801.
Guastella AR, Michelhaugh SK, Klinger NV, Kupsky WJ, Polin LA, Muzik O, Juhasz C, Mittal S. Tryptophan PET imaging of the kynurenine pathway in patient-derived xenograft models of glioblastoma. Mol Imaging. 2016;15
Li J, Deng H, Hu M, Fang Y, Vaughn A, Cai X, Xu L, Wan W, Li Z, Chen S, et al. Inhibition of non-small cell lung cancer (NSCLC) growth by a novel small molecular inhibitor of EGFR. Oncotarget. 2015;6(9):6749–61.
Oldrini B, Hsieh WY, Erdjument-Bromage H, Codega P, Carro MS, Curiel-Garcia A, Campos C, Pourmaleki M, Grommes C, Vivanco I, et al. EGFR feedback-inhibition by ran-binding protein 6 is disrupted in cancer. Nat Commun. 2017;8
Laramy JK, Kim M, Gupta SK, Parrish KE, Zhang S, Bakken KK, Carlson BL, Mladek AC, Ma DJ, Sarkaria JN, et al. Heterogeneous binding and CNS distribution of the multi-targeted kinase inhibitor ponatinib restrict orthotopic efficacy in a patient-derived xenograft model of glioblastoma. J Pharmacol Exp Ther. 2017;
Alifieris C, Trafalis DT. Glioblastoma multiforme: pathogenesis and treatment. Pharmacol Ther. 2015;152:63–82.
Huang PH, Mukasa A, Bonavia R, Flynn RA, Brewer ZE, Cavenee WK, Furnari FB, White FM. Quantitative analysis of EGFRvIII cellular signaling networks reveals a combinatorial therapeutic strategy for glioblastoma. Proc Natl Acad Sci U S A. 2007;104(31):12867–72.
Lal A, Glazer CA, Martinson HM, Friedman HS, Archer GE, Sampson JH, Riggins GJ. Mutant epidermal growth factor receptor up-regulates molecular effectors of tumor invasion. Cancer Res. 2002;62(12):3335–9.
Keller S, Schmidt MHH. EGFR and EGFRvIII promote angiogenesis and cell invasion in glioblastoma: combination therapies for an effective treatment. Int J Mol Sci. 2017;18(6)
Padfield E, Ellis HP, Kurian KM. Current therapeutic advances targeting EGFR and EGFRvIII in glioblastoma. Front Oncol. 2015;5:5.
Wikstrand CJ, Stanley SD, Humphrey PA, Pegram CN, Archer GE, Kurpad S, Shibuya M, Bigner DD. Investigation of a synthetic peptide as immunogen for a variant epidermal growth factor receptor associated with gliomas. J Neuroimmunol. 1993;46(1–2):165–73.
Wheeler DL, Dunn EF, Harari PM. Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat Rev Clin Oncol. 2010;7(9):493–507.
Taylor TE, Furnari FB, Cavenee WK. Targeting EGFR for treatment of glioblastoma: molecular basis to overcome resistance. Curr Cancer Drug Targets. 2012;12(3):197–209.
Ramakrishnan MS, Eswaraiah A, Crombet T, Piedra P, Saurez G, Iyer H, Arvind AS. Nimotuzumab, a promising therapeutic monoclonal for treatment of tumors of epithelial origin. MAbs. 2009;1(1):41–8.
Eskilsson E, Rosland GV, Solecki G, Wang Q, Harter PN, Graziani G, Verhaak RGW, Winkler F, Bjerkvig R, Miletic H. EGFR heterogeneity and implications for therapeutic intervention in glioblastoma. Neuro-Oncology. 2017;
An Z, Aksoy O, Zheng T, Fan QW, Weiss WA. Epidermal growth factor receptor and EGFRvIII in glioblastoma: signaling pathways and targeted therapies. Oncogene. 2018;37(12):1561–75.
Nair JJ, Bastida J, Codina C, Viladomat F, van Staden J. Alkaloids of the south African Amaryllidaceae: a review. Nat Prod Commun. 2013;8(9):1335–50.
Tamplin OJ, Durand EM, Carr LA, Childs SJ, Hagedorn EJ, Li P, Yzaguirre AD, Speck NA, Zon LI. Hematopoietic stem cell arrival triggers dynamic remodeling of the perivascular niche. Cell. 2015;160(1–2):241–52.
Yang Z, Guo Q, Wang Y, Chen K, Zhang L, Cheng Z, Xu Y, Yin X, Bai Y, Rabbie S, et al. AZD3759, a BBB-penetrating EGFR inhibitor for the treatment of EGFR mutant NSCLC with CNS metastases. Sci Transl Med. 2016;8(368):368ra172.
Acknowledgements
We thank Drs. Jie Tang and Weiliang Jiang for offering their insightful advice on this project.
Funding
This work was supported by the National Natural Science Foundation of China (No.81602649), National Natural Science Foundation of China (No. 81702985), Hubei Provincial Natural Science Foundation of China (No. 2016CFB210), Hubei Provincial Training Programs of Innovation and Entrepreneurship for Undergraduate (No. 201710927009Z) and the Startup Scientific Research Foundation for Doctors of Hubei University of Science and Technology (No. BK1506).
Availability of data and materials
The dataset generated or analyzed during this current study are available in PDB data base with the accession number cited in the article.
Author information
Authors and Affiliations
Contributions
JS and MH designed the research. JS, TZ, ZC, NZ, HW, LL, ZW, and HY conducted the experiments. JS and MH wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal experiments were approved by the Animal Center of Hubei University of Science and Technology and performed following International Guidelines and Protocols. The patients were informed about the study and were asked to sign consents for their tissues used in the scientific research, and this research also obtained approval from ethics of **anning central hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Shen, J., Zhang, T., Cheng, Z. et al. Lycorine inhibits glioblastoma multiforme growth through EGFR suppression. J Exp Clin Cancer Res 37, 157 (2018). https://doi.org/10.1186/s13046-018-0785-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13046-018-0785-4