Abstract
Background
Alzheimer’s disease (AD) is a chronic neurodegenerative disease with pathological hallmarks including the formation of extracellular aggregates of amyloid-beta (Aβ) known as plaques and intracellular tau tangles. Coincident with the formation of Aβ plaques is recruitment and activation of glial cells to the plaque forming a plaque niche. In addition to histological data showing the formation of the niche, AD genetic studies have added to the growing appreciation of how dysfunctional glia pathways drive neuropathology, with emphasis on microglia pathways. Genomic approaches enable comparisons of human disease profiles between different mouse models informing on their utility to evaluate secondary changes to triggers such as Aβ deposition.
Methods
In this study, we utilized two animal models of AD to examine and characterize the AD-associated pathology: the Tg2576 Swedish APP (KM670/671NL) and TgCRND8 Swedish plus Indiana APP (KM670/671NL + V717F) lines. We used laser capture microscopy (LCM) to isolate samples surrounding Thio-S positive plaques from distal non-plaque tissue. These samples were then analyzed using RNA sequencing.
Results
We determined age-associated transcriptomic differences between two similar yet distinct APP transgenic mouse models, known to differ in proportional amyloidogenic species and plaque deposition rates. In Tg2576, human AD gene signatures were not observed despite profiling mice out to 15 months of age. TgCRND8 mice however showed progressive and robust induction of lysomal, neuroimmune, and ITIM/ITAM-associated gene signatures overlap** with prior human AD brain transcriptomic studies. Notably, RNAseq analyses highlighted the vast majority of transcriptional changes observed in aging TgCRND8 cortical brain homogenates were in fact specifically enriched within the plaque niche samples. Data uncovered plaque-associated enrichment of microglia-related genes such as ITIM/ITAM-associated genes and pathway markers of phagocytosis.
Conclusion
This work may help guide improved translational value of APP mouse models of AD, particularly for strategies aimed at targeting neuroimmune and neurodegenerative pathways, by demonstrating that TgCRND8 more closely recapitulates specific human AD-associated transcriptional responses.
Similar content being viewed by others
Background
Alzheimer’s disease (AD) is a progressive, neurodegenerative disease characterized by a wide spectrum of pathological hallmarks including amyloid-beta (Aβ) plaques, intraneuronal neurofibrillary tangles, atrophy of synapses, and dendritic arbors, with associated cognitive decline [1]. While histological studies have characterized the morphology and process complexity of glial cells in a wide-range of neurodegenerative diseases, specific genomic and proteomic data regarding how microglia and astrocytes are modulated by disease, and vice versa, remain unknown. The lack of data regarding microglial reactivity in neurodegeneration is somewhat due to controversy regarding the utility of specific AD mouse models as windows into or prototype for human disease, and a paucity of studies using panoramic unbiased molecular profiling approaches to better describe local pathological changes occurring within the Aβ niche.
Numerous studies using mouse AD models have identified and pre-clinically validated mechanisms targeted at either neuro-inflammatory or innate immune pathways [2,3,4,5]. However, the predictive value of these data is predicated on the translational relevance of the selected mouse model. Hence, clear understanding of how molecular pathways, particularly those related to the neuroimmune response, change with respect to disease pathology in these models is a key to accurate data analysis and interpretation. RNAseq is well suited to map** out transcriptome changes across the entire genome and has significant advantages to low-throughout platforms and microarrays which are biased to known transcripts and generally remain limited to comparative inferences. One goal of the current study was to complete longitudinal evaluation of two different APP mouse models using RNAseq in brain regions demonstrating heavy plaque burden to evaluate their overlap with gene expression signatures previously identified in human AD brain samples.
Microglia are the brain’s resident immune cells that maintain central nervous system (CNS) homeostasis, constantly survey their environment, and react to injury by initiating an inflammatory reaction [6]. They express a diverse set of pattern recognition receptors capable of sensing these damage-associated signals and pathogens in the extracellular milieu and respond to neuronal injury and tissue pathogens by rapidly extending processes toward and chemotaxing into the pathological niche. Not surprisingly, in the AD brain, microglia cluster and surround Aβ plaques, a phenomenon also noted in many mouse models [7,8,9]. Despite the similar observations between mouse and humans, it remains to be seen how the plaque niche in AD mouse models may be dysregulated with regard to expression of human AD signatures, inflammatory versus innate immune pathways, and what transcriptome-wide profiling insights can be gained about biological changes occurring in the plaque-associated tissue. Notably, drugs targeting inflammation in AD, such as COX inhibitors and nonsteroidal anti-inflammatory drugs (NSAIDs), have failed in the clinic underscoring the need for validating translational relevance of mouse models with respect to neuroinflammation such that novel insights [10,11,12,13,14], therapeutic hypotheses, and targets are more likely to translate to clinical success.
Large-scale AD GWAS studies have provided genetic hypotheses for neuroinflammation in AD [15,16,17,18,19,20,21,22,3a). Conversely, the majority of the genes upregulated in the plaque versus non-plaque comparison were also upregulated in 6–10-month-old TgCRND8 animals compare to WT (Fig. 4b). Several genes (12,130) were detected in both experiments, including 342 genes that were upregulated in TgCRND8 vs WT cortex (p < 0.001 at any age) and 67 genes upregulated in plaque vs non-plaque (p < 0.001), of which 46 were overlap** between both signature sets (p < 2e-56) (Fig. 4c). We confirmed the findings from our RNAseq data using a customized NanoString nChip showing that key plaque-associated genes were upregulated (Trem2, Tyrobp, Cd68, Clec7a, Tspo, Itgfax) (Additional file 1: Figure S1). Data were analyzed for expression of a set of markers associated with resident microglia or peripheral macrophages as outlined by Hickman et al. to characterize the phenotype of cells surrounding the plaque [36]. Results show that 7 out of the top 25 most abundant genes in resident microglia were significantly upregulated in the plaque niche compared to non-plaque samples (Additional file 8: Figure S4). Conversely, only 1 gene associated with peripheral macrophage expression was significantly upregulated in the plaque niche, Complement C4-b (C4b) (Additional file 8: Figure S4). This phenotypic analysis was more pronounced in whole cortex samples resulting in 13 out of the top 25 microglial-specific genes were significantly upregulated in TgCRND8 mice compared to WT compared to only 1 gene in the peripheral macrophage signature (Additional file 8: Figure S4).
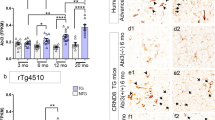
Localization of TREM2 and Cd33 around amyloid-beta (Aβ) plaques in the aged TgCRND8 mouse. Brain slices were prepared from 35-week-old wild-type and TgCRND8 mice and processed for amyloid-beta immunohistochemistry (6E10 labeling, magenta) combined with TREM2 (panel a) or CD33 (panel b) in situ hybridization via RNAScope (brown). Visualization of TREM2 and Cd33 confirms their associated expression pattern with Aβ pathology, supporting the rationale for laser capture microdissection of Thio-S-labeled plaques for transcriptome-wide RNA sequencing. Representative images show section sampling for LCM and RNA seq (c, d). Images show Iba1 (brown) and 6E10 (magenta) immunostainings. Scale bars, 50 and 100 μm
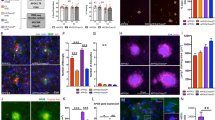
Plaque-niche gene expression differences in 6-month-old TgCRND8. a Student’s t test p value distributions for gene expression differences plaque and normal samples in TgCRND8 cortex at 6 months of age. Gray line indicates expected false discovery rate (FDR) given multiple test comparisons. b Heatmap showing log10 ratio values from each sample (y-axis) for each gene (x-axis) with t test p value < 0.001 between plaque and normal tissue in TgCRND8 cortex. Samples are ordered manually by genotype as indicated. Genes are ordered by agglomerative clustering. c Heatmaps showing log10 ratio values from each sample (y-axis) for each gene (x-axis) within the indicated gene sets. Samples are ordered manually by genotype as indicated. Genes are ordered by agglomerative clustering within each set
Overlap of TgCRND8 progression signature and plaque-niche signature. a Heatmap showing log10 ratio values from each LCM sample (y-axis) for each gene (x-axis) detected in both the LCM and whole cortex studies and with t test p < 0.001 between TgCRND8 and WT cortex at one or more ages. Samples are ordered manually by genotype as indicated. Genes are ordered by agglomerative clustering. b Heatmap showing log10 ratio values from each whole cortex sample (y-axis) for each gene (x-axis) detected in both the whole cortex and LCM studies and with t test p < 0.001 between plaque and normal tissue in TgCRND8 cortex. Samples are ordered manually by genotype as indicated. Genes are ordered by agglomerative clustering. c Venn diagram depicting number of genes in common between signatures in a and b, and the associated hypergeometric p value given 12,130 genes detected in both experiments
To further characterize the microglia phenotype observed in the TgCRND8 plaque niche, we compared the gene signatures identified here to those of the microglial subtype, disease-associated microglia (DAM), recently shown to surround plaques in another mouse AD model expressing five familiar AD mutations (5XFAD) [37]. Indeed, genes reported to be expressed higher in the DAM population (> 1.5 fold, p < 1e-5) were strongly enriched among the upregulated gene signatures in both the TgCRND8 whole cortex and the LCM-dissected TgCRND8 plaque tissue signatures (p = 4e-25 and p = 8e-13, respectively) (Fig. 5a–d; Additional file 4: Table S2, Additional file 6: Table S3, and Additional file 7: Table S4). Across all three gene sets, eight genes were commonly expressed: Ccl6, Cd9, Ctsz, Gusb, Lyr2, Npc2, Trem2, and Tyrobp. In contrast, the DAM marker genes were not significantly represented among the Tg2576-related signatures (p = 0.3) (Fig. 5a, c). In additional analyses, expression of markers of M1 and M2 microglia as outlined in [38, 39] were also analyzed for differential expression in plaque vs. non-plaque samples as well as in whole cortex samples from TgCRND8 compared to WT controls. Results show a significant increase in expression of only one marker of M1 activation, CD16a, in the plaque niche compared to non-plaque controls (3.06-fold; p < 0.0001). No markers of M2 activation were significantly upregulated in plaque samples compared to non-plaque. Whole cortex TgCRND8 displayed significant (p < 0.001) expression of four markers of M1 activation, CXCL10 (34.52-fold; p < 0.0001), CCR5 (1.51-fold; p < 0.0001), CD86 (2.25-fold; p < 0.0001), and CD16a (2.62-fold; p < 0.0001) in TgCNRD8 compared to WT, and only two markers of M2 activation/repair, Clec7a (72.33-fold; p < 0.0001), and TGFβ (2.23-fold; p < 0.0001).
Overlap of TgCRND8 progression signature and plaque-niche signature. a, b Heatmap showing log10 ratio values from each whole cortex sample (a) or each LCM sample (b) for genes reported to be expressed higher (> 1.5 fold, p < 1e-5) in the DAM population [37]. Samples are ordered manually by genotype and age as indicated. Genes are ordered by agglomerative clustering. c Venn diagram depicting number of genes in common between the Tg576/TgCRND8 whole cortex signatures (p < 0.001, any age) and the genes expressed higher (> 1.5 fold, p < 1e-5) in the DAM cells. d Venn diagram depicting number of genes in common between the TgCRND8 LCM plaque signature (p < 0.001) and the genes expressed higher (> 1.5 fold, p < 1e-5) in the DAM population
Discussion
Many studies have demonstrated a strong association between inflammation, innate immunity, and amyloid plaques in both human AD and preclinical models [5c). Thus, our data suggests that the TgCRND8 model is a more relevant model to understand polarization states and plaque niche.
The TgCRND8 transcriptional profiles align with previous findings of robust transcriptional responses in recently discovered genetic links between microglia, innate immunity, and AD. Numerous GWAS and meta-analyses in various AD cohorts’ studies have identified novel microglial-associated receptors TREM2 and CD33 for AD [16, 18, 29]. This has naturally led to investigations of their roles in AD in preclinical models. In the current study, regional assessment of the Aβ plaque niche revealed TREM2 and its signaling adaptor TYROBP (which encodes the DAP12 protein) are significantly (p < 0.001) upregulated in the Aβ plaque niche compared to non-plaque areas. Both markers show a 3-fold increase in expression near the plaque compared to non-plaque samples. An expanded view beyond these hub genes confirms that several ITIM/ITAM-associated transcripts are also enriched around plaques. In TgCRND8 cortical homogenates, this innate immune panel was shown to be upregulated at 6 and 10 months of age, as well. It is important to highlight, however, that the ITIM/ITAM-associated module is not invariably associated with Aβ plaque pathology. In the Tg2576 model, we did not observe significant induction of this AD-implicated innate immune signature. Therefore, when juxtaposed with robust progressive signature induction in the TgCNRD8 mice, it is clear that not all mouse APP models are equivalent in this regard.
Exploring more closely specific ITIM/ITAM-associated changes, we identified novel dysregulated pathway nodes as potential modulatory factors, including genes known to be enriched in microglia such as receptor and non-receptor tyrosine kinases, as well as associated phosphatases predicted to inhibit phagocytic signal transduction. For instance, it is widely known that DAP12 signals via SYK induction and its downstream signaling may be negatively regulated by various phosphatases such as PTPN6 and INPP5D. Interestingly, not only do we observe robust SYK upregulation around amyloid plaques (> 3-fold) but associated protein tyrosine phosphatases PTPN6 and INPP5D are also enriched (upregulated 6.0-fold and 2.7-fold respectively). Both PTPN6 and INPP5D are involved in the signaling via inhibitory receptors found in innate immune cells, such as CD33 for example. Moreover, in a meta-analysis of major AD GWAS, the rs35349669 locus on chromosome 2 encoding INPP5D was recently identified as a novel AD risk-associated locus [60,61,62]. Within the plaque niche, we also observed that several inhibitory ITIM-containing receptors in addition to CD33 are also enriched, such as Slamf9 (7.8-fold, p = 0.011), FCGR1 (2.2-fold, p = 0.002), and LAIR1 (2.0-fold, p = 0.001). Likewise, we identified several additional ITAM-associated receptors such as FCGR1 (2.2-fold, p = 0.002), FCGR3 (3.1-fold, p = 1.4e-06), and CSF1R (1.5-fold, p = 0.0013) are significantly increased in the plaque niche compared to non-plaque areas.
Functional studies have begun to identify a role for the TREM2/DAP12 pathway in promoting cell survival and phagocytic capacity [63]. TREM2 deficiency reportedly impairs the phagocytosis of apoptotic neurons in microglia [64], possibly through apolipoprotein E and lipid-sensing mechanisms [65,66,67,68]. Similarly, in primary microglia isolated from TREM2 knockout mice, impaired phagocytic capacity of microbeads as well as Escherichia coli bacteria imply that TREM2 plays an important regulatory role in innate immune activity and possibly host defense mechanism [69]. Similar functional insights have been reported for CD33. A study by Gricuic et al. observed increased expression of CD33 positive microglia in AD brains, a significant positive correlation between Aβ pathology and CD33 expression as well as an increase in microglial uptake of Aβ in vitro after CD33 inactivation [32]. Interestingly, clinical data show an increase in PiB imaging in patients with the risk allele for CD33 [16], further demonstrating a relationship with amyloid pathology and potential to track the progression of disease-related PET-imaging biomarkers. These findings serve as the foundation for the therapeutic hypothesis that blockade of CD33 function could be a compelling alternative to accelerate Aβ clearance [32]. Thus, when considering collectively the functional roles for TREM2 and CD33 in microglia and AD, it appears that converging and opposing innate immune regulation may exist and dictate risk status for human AD.
Conclusions
The current study highlights the importance of careful consideration and selection of appropriate APP mouse models specifically for exploring neuroimmune modulation, in particular ITIM/ITAM-associated mechanisms, such as TREM2 and CD33. Combined with the insights from human genetics, these findings provide broad evidence for the presence of a complex relationship between activating and inhibitory mechanisms which collectively may determine innate immune status around the Aβ plaque niche. Future studies utilizing single-cell profiling of microglia in AD mouse models to further characterize the co-expression of immune activating (e.g., ITAM) and suppressing (e.g., ITIM) mechanisms and will help to define how the underlying regulation of mechanisms controlling this microglial innate immune rheostat are shaped by neuropathology. Such studies will also help clarify whether distinct heterogeneous sub-populations of microglia exist around amyloid plaques, or whether competing inhibitory and activating nodes are commonly present within individual plaque-associated microglia. The observations provide tissue-level insights into plaque-associated transcriptional signatures to encourage more fine characterization of cell-specific pathway changes across such broad panels of markers.
Abbreviations
- AD:
-
Alzheimer’s disease
- APP:
-
Amyloid precursor protein
- Aβ:
-
Amyloid-beta
- CNS:
-
Central nervous system
- DAM:
-
Disease-associated microglia
- GWAS:
-
Genome-wide association studies
- ITAM:
-
Immunoreceptor tyrosine-based activation motif
- ITIM:
-
Immunoreceptor tyrosine-based inhibition motif
- LOAD:
-
Late-onset Alzheimer’s disease
References
Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009;118:5–36.
Guillot-Sestier MV, Doty KR, Town T. Innate immunity fights Alzheimer’s disease. Trends Neurosci. 2015;38:674–81.
Yan R, Vassar R. Targeting the beta secretase BACE1 for Alzheimer’s disease therapy. Lancet Neurol. 2014;13:319–29.
Orre M, Kamphuis W, Osborn LM, Jansen AHP, Kooijman L, Bossers K, Hol EM. Isolation of glia from Alzheimer’s mice reveals inflammation and dysfunction. Neurobiol Aging. 2014;35:2746–60.
Gjoneska E, Pfenning AR, Mathys H, Quon G, Kundaje A, Tsai LH, Kellis M. Conserved epigenomic signals in mice and humans reveal immune basis of Alzheimer’s disease. Nature. 2015;518:365–9.
Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–8.
Ager RR, Fonseca MI, Chu SH, Sanderson SD, Taylor SM, Woodruff TM, Tenner AJ. Microglial C5aR (CD88) expression correlates with amyloid-beta deposition in murine models of Alzheimer’s disease. J Neurochem. 2010;113:389–401.
Arends YM, Duyckaerts C, Rozemuller JM, Eikelenboom P, Hauw JJ. Microglia, amyloid and dementia in alzheimer disease. A correlative study. Neurobiol Aging. 2000;21:39–47.
Rodriguez GA, Tai LM, LaDu MJ, Rebeck GW. Human APOE4 increases microglia reactivity at Abeta plaques in a mouse model of Abeta deposition. J Neuroinflammation. 2014;11:111.
Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, Farlow MR, ** S, Thomas RG, Thal LJ. Alzheimer’s disease cooperative S: effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289:2819–26.
Aisen PS, Schmeidler J, Pasinetti GM. Randomized pilot study of nimesulide treatment in Alzheimer’s disease. Neurology. 2002;58:1050–4.
Green RC, Schneider LS, Amato DA, Beelen AP, Wilcock G, Swabb EA, Zavitz KH. Tarenflurbil phase 3 study G: effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA. 2009;302:2557–64.
Pasqualetti P, Bonomini C, Dal Forno G, Paulon L, Sinforiani E, Marra C, Zanetti O, Rossini PM. A randomized controlled study on effects of ibuprofen on cognitive progression of Alzheimer’s disease. Aging Clin Exp Res. 2009;21:102–10.
Reines SA, Block GA, Morris JC, Liu G, Nessly ML, Lines CR, Norman BA, Baranak CC. Rofecoxib protocol 091 study G: Rofecoxib: no effect on Alzheimer’s disease in a 1-year, randomized, blinded, controlled study. Neurology. 2004;62:66–71.
Bettens K, Sleegers K, Van Broeckhoven C. Genetic insights in Alzheimer’s disease. Lancet Neurol. 2013;12:92–104.
Bradshaw EM, Chibnik LB, Keenan BT, Ottoboni L, Raj T, Tang A, Rosenkrantz LL, Imboywa S, Lee M, Von Korff A, et al. CD33 Alzheimer’s disease locus: altered monocyte function and amyloid biology. Nat Neurosci. 2013;16:848–50.
Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, Abraham R, Hamshere ML, Pahwa JS, Moskvina V, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet. 2011;43:429–35.
Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med. 2013;368:107–16.
Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41:1094–9.
Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, DeStafano AL, Bis JC, Beecham GW, Grenier-Boley B, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45:1452–8.
Seshadri S, Fitzpatrick AL, Ikram MA, DeStefano AL, Gudnason V, Boada M, Bis JC, Smith AV, Carassquillo MM, Lambert JC, et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303:1832–40.
Villegas-Llerena C, Phillips A, Garcia-Reitboeck P, Hardy J, Pocock JM. Microglial genes regulating neuroinflammation in the progression of Alzheimer’s disease. Curr Opin Neurobiol. 2016;36:74–81.
Zhang B, Gaiteri C, Bodea LG, Wang Z, McElwee J, Podtelezhnikov AA, Zhang C, **e T, Tran L, Dobrin R, et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell. 2013;153:707–20.
Efthymiou AG, Goate AM. Late onset Alzheimer’s disease genetics implicates microglial pathways in disease risk. Mol Neurodegener. 2017;12:43.
Bianchin MM, Capella HM, Chaves DL, Steindel M, Grisard EC, Ganev GG, da Silva Junior JP, Neto Evaldo S, Poffo MA, Walz R, et al. Nasu-Hakola disease (polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy––PLOSL): a dementia associated with bone cystic lesions. From clinical to genetic and molecular aspects. Cell Mol Neurobiol. 2004;24:1–24.
Chouery E, Delague V, Bergougnoux A, Koussa S, Serre JL, Megarbane A. Mutations in TREM2 lead to pure early-onset dementia without bone cysts. Hum Mutat. 2008;29:E194–204.
Giraldo M, Lopera F, Siniard AL, Corneveaux JJ, Schrauwen I, Carvajal J, Munoz C, Ramirez-Restrepo M, Gaiteri C, Myers AJ, et al. Variants in triggering receptor expressed on myeloid cells 2 are associated with both behavioral variant frontotemporal lobar degeneration and Alzheimer’s disease. Neurobiol Aging. 2013;2077(34):e2011–78.
Montalbetti L, Ratti MT, Greco B, Aprile C, Moglia A, Soragna D. Neuropsychological tests and functional nuclear neuroimaging provide evidence of subclinical impairment in Nasu-Hakola disease heterozygotes. Funct Neurol. 2005;20:71–5.
Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, et al. TREM2 variants in Alzheimer’s disease. N Engl J Med. 2013;368:117–27.
Condello C, Yuan P, Schain A, Grutzendler J. Microglia constitute a barrier that prevents neurotoxic protofibrillar Abeta42 hotspots around plaques. Nat Commun. 2015;6:6176.
Frank S, Burbach GJ, Bonin M, Walter M, Streit W, Bechmann I, Deller T. TREM2 is upregulated in amyloid plaque-associated microglia in aged APP23 transgenic mice. Glia. 2008;56:1438–47.
Griciuc A, Serrano-Pozo A, Parrado AR, Lesinski AN, Asselin CN, Mullin K, Hooli B, Choi SH, Hyman BT, Tanzi RE. Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron. 2013;78:631–43.
Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102.
Podtelezhnikov AA, Tanis KQ, Nebozhyn M, Ray WJ, Stone DJ, Loboda AP. Molecular insights into the pathogenesis of Alzheimer’s disease and its relationship to normal aging. PLoS One. 2011;6:e29610.
Liao L, Cheng D, Wang J, Duong DM, Losik TG, Gearing M, Rees HD, Lah JJ, Levey AI, Peng J. Proteomic characterization of postmortem amyloid plaques isolated by laser capture microdissection. J Biol Chem. 2004;279:37061–8.
Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang LC, Means TK, El Khoury J. The microglial sensome revealed by direct RNA sequencing. Nat Neurosci. 2013;16:1896–905.
Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell. 2017;169:1276–90. e1217
Aggarwal NR, King LS, D'Alessio FR. Diverse macrophage populations mediate acute lung inflammation and resolution. Am J Physiol Lung Cell Mol Physiol. 2014;306:L709–25.
Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, et al. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17:131–43.
Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405.
Mullan M, Crawford F, Axelman K, Houlden H, Lilius L, Winblad B, Lannfelt L. A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N-terminus of beta-amyloid. Nat Genet. 1992;1:345–7.
Bornemann KD, Staufenbiel M. Transgenic mouse models of Alzheimer’s disease. Ann N Y Acad Sci. 2000;908:260–6.
Sasaguri H, Nilsson P, Hashimoto S, Nagata K, Saito T, De Strooper B, Hardy J, Vassar R, Winblad B, Saido TC. APP mouse models for Alzheimer’s disease preclinical studies. EMBO J. 2017;36:2473–87.
Gu L, Guo Z. Alzheimer’s Abeta42 and Abeta40 peptides form interlaced amyloid fibrils. J Neurochem. 2013;126:305–11.
Jan A, Gokce O, Luthi-Carter R, Lashuel HA. The ratio of monomeric to aggregated forms of Abeta40 and Abeta42 is an important determinant of amyloid-beta aggregation, fibrillogenesis, and toxicity. J Biol Chem. 2008;283:28176–89.
Kuperstein I, Broersen K, Benilova I, Rozenski J, Jonckheere W, Debulpaep M, Vandersteen A, Segers-Nolten I, Van Der Werf K, Subramaniam V, et al. Neurotoxicity of Alzheimer’s disease Abeta peptides is induced by small changes in the Abeta42 to Abeta40 ratio. EMBO J. 2010;29:3408–20.
Pauwels K, Williams TL, Morris KL, Jonckheere W, Vandersteen A, Kelly G, Schymkowitz J, Rousseau F, Pastore A, Serpell LC, Broersen K. Structural basis for increased toxicity of pathological abeta42:abeta40 ratios in Alzheimer disease. J Biol Chem. 2012;287:5650–60.
Snyder SW, Ladror US, Wade WS, Wang GT, Barrett LW, Matayoshi ED, Huffaker HJ, Krafft GA, Holzman TF. Amyloid-beta aggregation: selective inhibition of aggregation in mixtures of amyloid with different chain lengths. Biophys J. 1994;67:1216–28.
Yoshiike Y, Chui DH, Akagi T, Tanaka N, Takashima A. Specific compositions of amyloid-beta peptides as the determinant of toxic beta-aggregation. J Biol Chem. 2003;278:23648–55.
McGowan E, Pickford F, Kim J, Onstead L, Eriksen J, Yu C, Skipper L, Murphy MP, Beard J, Das P, et al. Abeta42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47:191–9.
Frautschy SA, Yang F, Irrizarry M, Hyman B, Saido TC, Hsiao K, Cole GM. Microglial response to amyloid plaques in APPsw transgenic mice. Am J Pathol. 1998;152:307–17.
Gyoneva S, Swanger SA, Zhang J, Weinshenker D, Traynelis SF. Altered motility of plaque-associated microglia in a model of Alzheimer’s disease. Neuroscience. 2016;330:410–20.
Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol. 1989;24:173–82.
Perlmutter LS, Barron E, Chui HC. Morphologic association between microglia and senile plaque amyloid in Alzheimer’s disease. Neurosci Lett. 1990;119:32–6.
Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49:489–502.
Stalder M, Phinney A, Probst A, Sommer B, Staufenbiel M, Jucker M. Association of microglia with amyloid plaques in brains of APP23 transgenic mice. Am J Pathol. 1999;154:1673–84.
Yin Z, Raj D, Saiepour N, Van Dam D, Brouwer N, Holtman IR, Eggen BJL, Moller T, Tamm JA, Abdourahman A, et al. Immune hyperreactivity of Abeta plaque-associated microglia in Alzheimer’s disease. Neurobiol Aging. 2017;55:115–22.
Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol. 2010;10:453–60.
Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15:300–12.
Farfel JM, Yu L, Buchman AS, Schneider JA, De Jager PL, Bennett DA. Relation of genomic variants for Alzheimer disease dementia to common neuropathologies. Neurology. 2016;87:489–96.
Schott JM, Crutch SJ, Carrasquillo MM, Uphill J, Shakespeare TJ, Ryan NS, Yong KX, Lehmann M, Ertekin-Taner N, Graff-Radford NR, et al. Genetic risk factors for the posterior cortical atrophy variant of Alzheimer’s disease. Alzheimers Dement. 2016;12:862–71.
Chouraki V, Reitz C, Maury F, Bis JC, Bellenguez C, Yu L, Jakobsdottir J, Mukherjee S, Adams HH, Choi SH, et al. Evaluation of a genetic risk score to improve risk prediction for Alzheimer’s disease. J Alzheimers Dis. 2016;53:921–32.
Painter MM, Atagi Y, Liu CC, Rademakers R, Xu H, Fryer JD, Bu G. TREM2 in CNS homeostasis and neurodegenerative disease. Mol Neurodegener. 2015;10:43.
Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201:647–57.
Atagi Y, Liu CC, Painter MM, Chen XF, Verbeeck C, Zheng H, Li X, Rademakers R, Kang SS, Xu H, et al. Apolipoprotein E is a ligand for triggering receptor expressed on myeloid cells 2 (TREM2). J Biol Chem. 2015;290:26043–50.
Bailey CC, DeVaux LB, Farzan M. The triggering receptor expressed on myeloid cells 2 binds apolipoprotein E. J Biol Chem. 2015;290:26033–42.
Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, Gilfillan S, Krishnan GM, Sudhakar S, Zinselmeyer BH, et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell. 2015;160:1061–71.
Yeh FL, Wang Y, Tom I, Gonzalez LC, Sheng M. TREM2 binds to apolipoproteins, including APOE and CLU/APOJ, and thereby facilitates uptake of amyloid-beta by microglia. Neuron. 2016;91:328–40.
Kleinberger G, Yamanishi Y, Suarez-Calvet M, Czirr E, Lohmann E, Cuyvers E, Struyfs H, Pettkus N, Wenninger-Weinzierl A, Mazaheri F, et al. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci Transl Med. 2014;6:243ra286.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon acceptance.
Author information
Authors and Affiliations
Contributions
SMR contributed to study design, performed the experiments, and contributed to writing of the manuscript. KQT contributed to study design, performed statistical analysis, and contributed to writing of the manuscript. PG and JM aided with project execution and edited the manuscript. VM and JG carried out bioinformatics and analysis. MP, RS, and VM prepared samples. CW provided in situ histological samples, imaging, and analysis. EOL contributed to writing of the manuscript, figure construction, and carried analysis. GZ contributed to histological data. RS prepared the samples for LCM experiments and data analysis. BJH, JK, and MK all critically reviewed and edited the manuscript. CM supervised the project and contributed to the study design, experiments, analysis, and writing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Principles of laboratory animal care were followed, and all studies were previously approved by the Institutional Animal Care and Use Committee and were performed in accordance to the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health (Library of Congress Control Number 2010940400, revised 2011).
Consent for publication
Not applicable
Competing interests
All authors were or are employees and shareholders in Merck & Co., Inc. at the time of their contributions. The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Figure S1. Nanostring confirmation of plaque associate selected gene in TgCRND8. Selected plaque associated genes that were upregulated in LCM tissue in TgCRND8 mice by RNAseq were confirmed using a Nanostring customized chip (n=4 per group). Bar graphs show nCounts (mean ± s.e.m) to highlight changes for a subset of specific transcripts included for direct comparison. *p < 0.05, **p < 0.01, by student t-test, 2-tailed. (JPG 51 kb)
Additional file 2:
Figure S2. Nanostring confirmation of plaque associate selected gene in TgCRND8. Selected plaque associated genes that were upregulated in LCM tissue in TgCRND8 mice by RNA seq confirmed using Nanostring customized chip (n=4 per group). Dot plots show mRNAs transcripts (mean ± s.e.m). *p < 0.05, **p < 0.01, ***p < 0.001, by student t-test, 2-tailed. (JPG 175 kb)
Additional file 3:
Table S1. Age and strain-related transcriptomic changes in AD models. RNAseq was carried out on cortexes isolated from Tg2576 (ages: 3mo, 6mo, 9mo, 12mo and 15mo, respectively), TgCRND8 (1.5mo, 3mo, 4.5mo, 6mo and 10mo, respectively) and WT age-matched controls mice. Data shows statistical analysis of age correlation between each strain, p-value and log10 analysis. Differentially expressed genes were identified by Pearson correlation or T-test using Matlab R2010b (Mathworks). A p-value cutoff of < 0.001 was used to identify differentially expressed genes. The FDR corresponding to this p-value is given in each of the comparisons to convey relative signature confidence. Set annotation analysis was performed by comparing input sets to GeneGo (www.genego.com), Ingenuity (www.ingenuity.com) and KEGG (www.genome.jp/kegg/) pathway sets. Bonferroni corrected hypergeometric p-values expectation (e)-values) of less than 0.1 were considered significant overlap between sets. (XLS 7280 kb)
Additional file 4:
Table S2. Age and strain-related pathway analysis in TgCRND8 cortex. Pathways analysis showing data from upregulated network processes in TgCRND8 cortex and plaque-associated tissue. Differentially expressed genes were identified by Pearson correlation or T-test using Matlab R2010b (Mathworks). A p-value cutoff of < 0.001 was used to identify differentially expressed genes. The FDR corresponding to this p-value is given in each of the comparisons to convey relative signature confidence. Set annotation analysis was performed by comparing input sets to GeneGo (www.genego.com), Ingenuity (www.ingenuity.com) and KEGG (www.genome.jp/kegg/) pathway sets. Bonferroni corrected hypergeometric p-values (expectation (e)-values) of less than 0.1 were considered significant overlap between sets. (XLSX 81 kb)
Additional file 5:
Figure S3. Tg2576 brain sections illustrating amyloid-beta pathology. Representative images of plaque pathology in 15-month old transgenic mice via anti-amyloid-β, 1-12 (26D6). (JPG 183 kb)
Additional file 6:
Table S3. Human AD translational transcriptomic profiling. Comparative gene network analysis was carried out from the mouse models used (tgCRND8 and Tg2576 models) in our study with that of: 1) Human aging (up-regulated) 2) Human aging (down-regulated) 3) Human inflammation signature 3) ITIM/ITAM-domain associated network signature 3) Mouse microglial gene signature (Barres et al., 2013) and 4) Mouse disease-associated microglia or DAM gene signature (Keren-Sheul et al., 2017). Differentially expressed genes were identified by Pearson correlation or T-test using Matlab R2010b (Mathworks). A. p-value cutoff of < 0.001 was used to identify differentially expressed genes. The FDR corresponding to this p-value is given in each of the comparisons to convey relative signature confidence. Set annotation analysis was performed by comparing input sets to GeneGo (www.genego.com), Ingenuity (www.ingenuity.com) and KEGG (www.genome.jp/kegg/) pathway sets. Bonferroni corrected hypergeometric p-values (expectation (e)-values) of less than 0.1 were considered significant overlap between sets. (XLSX 26 kb)
Additional file 7:
Table S4. Laser captured plaque transcriptomic signature. RNAseq data of laser-capture microscopy of TgCRND8 plaques versus non-plaque tissue as outlined in schematic in Fig. 2. Data shows statistical analysis of TgCRND8 plaques versus non-plaque tissue at 6 months of age (p-value) and log10 analysis of the ratio of TgCRND8 plaques versus non-plaque tissue. Differentially expressed genes were identified by Pearson correlation or T-test using Matlab R2010b (Mathworks). A p-value cutoff of < 0.001 was used to identify differentially expressed genes. The FDR corresponding to this p-value is given in each of the comparisons to convey relative signature confidence. Set annotation analysis was performed by comparing input sets to GeneGo (www.genego.com), Ingenuity (www.ingenuity.com) and KEGG (www.genome.jp/kegg/) pathway sets. Bonferroni corrected hypergeometric p-values (expectation (e)-values) of less than 0.1 were considered significant overlap between sets. (XLS 1410 kb)
Additional file 8:
Figure S4. Expression of microglial versus peripheral macrophage markers. Data were analyzed for fold changes in genes associated with either resident microglia (A) or peripheral macrophages (B) as outlined by Hickman et al., 2013. Results demonstrate significant (*p < 0.001) expression of 7 out of the top 25 most abundant genes in microglia in plaque samples compared to non-plaque controls whereas only 1 gene, C4b, of genes associated with peripheral macrophages displayed significant expression in plaque compared to non-plaque samples. For whole cortex samples, half of the top 25 microglial genes were significantly increased in plaque samples compared to non-plaque. (JPG 43 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Rothman, S.M., Tanis, K.Q., Gandhi, P. et al. Human Alzheimer’s disease gene expression signatures and immune profile in APP mouse models: a discrete transcriptomic view of Aβ plaque pathology. J Neuroinflammation 15, 256 (2018). https://doi.org/10.1186/s12974-018-1265-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12974-018-1265-7