Abstract
Background
Amyotrophic lateral sclerosis (ALS) is a fatal multifactorial neurodegenerative disease characterized by selective death of motor neurons in the cortex, brainstem, and spinal cord. Cytosolic phospholipase A2 alpha (cPLA2α) upregulation and activation in the spinal cord of patients with sporadic ALS and in the spinal cord of human mutant SOD1G93A (hmSOD1) transgenic mice were recently reported.
Methods
cPLA2α upregulation in the brainstem and spinal cord was reduced by brain infusion of a specific antisense oligonucleotide against cPLA2α (AS), and the effect was evaluated on disease progression and brain cell activation.
Results
We found that the elevation of cPLA2α protein expression in the spinal cord was first detected at 6-week-old hmSOD1 mice and remained elevated during their whole life span. Reduction of the elevated expression of cPLA2α in the spinal cord of hmSOD1 mice by brain infusion of an AS at week 15 (shortly before the appearance of the disease symptoms), for a duration of 6 weeks, delayed the loss of motor neuron function in comparison with hmSOD1 mice and with sense brain-infused hmSOD1 mice. To characterize the effect of cPLA2α upregulation on different processes taking place at the appearance of the disease symptoms, mice were brain infused with AS or with sense at week 15 for 3–4 weeks. The AS treatment that reduced cPLA2α upregulation in the spinal cord of AS-treated hmSOD1 mice (as analyzed at week 18–19) prevented the reduction in the number of the neurons (detected by NeuN) and inhibited astrocyte activation (detected by GFAP) and microglia activation (detected by Iba-1 and by CD40). In addition, AS treatment blunted the upregulation of the proinflammatory enzyme-inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) detected in hmSOD1 mice.
Conclusions
Since specific reduction of cPLA2α in the brainstem and spinal cord significantly attenuated the development of the disease, cPLA2α may offer an efficient target for treatment of ALS.
Similar content being viewed by others
Background
Amyotrophic lateral sclerosis (ALS) is a severe degenerative disorder, mainly affecting the motor neurons in the spinal cord, brainstem, and cortex. Most of the cases (about 90 %) are sporadic. Familial cases have been linked to mutations in a number of genes, including Cu/Zn superoxide dismutase (SOD1), TAR DNA binding protein (TDP-43), and chromosome 9 open reading frame 72 (C9ORF72) repeat expansions [1]. Mutant SOD1 is the best characterized form of familial ALS, accounting for 15–20 % of familial cases [2]. The etiology of sporadic ALS is unknown, although it is generally believed that sporadic and familial ALS may share pathological mechanisms. The pathophysiology of the multifactorial-multisystemic ALS disease includes various mechanisms. Among them are oxidative damage, high levels of glutamate, a reduced secretion of neurotrophic factors, protein aggregations, malfunctioning of the mitochondria, rupture in the axonal passage, destruction in the calcium metabolism, and changes in the skeletal proteins [3]. Although ALS is not primarily considered an inflammatory or immune-mediated disease, immune mechanisms appear to play a role in the pathogenesis of the disease. In both ALS patients and animal models, inflammatory responses have been observed [3–5]. Microglia [26].
End-stage analysis
To determine the mortality in a reliable and human fashion, end stage was defined as the inability of the mice to right themselves 30 s after being placed on one of their sides.
Statistical analysis
Data were expressed as mean ± standard error of the mean (SEM). Significance was determined by either one- or two-way analysis of variance (ANOVA) followed by a posteriori Bonferroni’s test for multiple comparisons provided by GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, USA). Survival was evaluated by Kaplan-Meier analysis.
Results
In order to study the role of cPLA2α in the development of ALS, cPLA2α protein expression was analyzed in the spinal cord of hmSOD1 mice (Fig. 1a) at different stages during their life span (Fig. 1b). Immunofluorescence staining and quantitation showed a significant (p < 0.001) elevation of cPLA2α protein expression in the spinal cord (Fig. 1a) of 6-week-old hmSOD1 mice, before the loss of motor neuronal function that appeared around week 17 (Fig. 1c). cPLA2α upregulation was also detected in the mice brainstem from week 6 onward and showed similar results (not shown). Neuronal or glial cells were stained by specific markers in the spinal cord of hmSOD1 mice at the same time points (Fig. 1d). Immunofluorescence staining and quantitation of neurons in the spinal cord using anti-NeuN showed a significant (p < 0.001) reduction in the neuron cell area from week 19 that was further reduced at the end stage. The neurons were counted according to their size and we found a similar reduction of around 40 % at 19 weeks and around 60 % at end stage in each group, suggesting that the reduction in cell area is due to both the number and the size of the neurons. A significant (p < 0.001) appearance of activated microglia (M1 phenotype) could be detected in the spinal cord of 15-week-old hmSOD1 mice by Iba-1 or CD40 staining that was gradually increased with age. The activation of microglia at week 15 preceded neuronal damage (Fig. 1d) and the appearance of motor neuron dysfunction (Fig. 1c). Activated astrocytes determined by GFAP were significantly (p < 0.001) detected in the spinal cord of 19-week-old mSOD1 mice and were dramatically increased at the end stage. Double staining with Iba-1 and anti-CD169 for the detection of monocytes revealed a significant (p < 0.001) appearance of monocytes in the spinal cord only at the end stage (Fig. 1e). As shown by confocal microscopy in Fig. 2a and in the inset Fig. 2b, double staining analysis of cPLA2α and the different cell types suggests that the main cells expressing cPLA2α protein are the neurons. High levels of cPLA2α protein were detected in the large neurons present in the spinal cord of mSOD1 mice at 6 and 11 weeks and in the small and damaged neurons at 19 weeks and end stage. The slight reduction of cPLA2α protein expression at the end stage (Fig. 1a) is probably due to the reduced number and size of the neurons (Fig. 1d) which are the main cells expressing high levels of cPLA2α. Confocal microscopy of higher magnification of the double staining analysis of cPLA2α and Iba-1 or GFAP showed that elevated cPLA2α could be detected in activated microglia and astrocytes (Fig. 2b). Confocal microscopy of double staining of the activated form of cPLA2α, phospho-cPLA2α on serine 505 (pcPLA2α), and the markers of the different cell types (Fig. 2c) showed that pcPLA2α was detected in the nuclei of the cells.
cPLA2α protein levels in the spinal cord of hmSOD1 mice during the development of the disease in hmSOD1 mice. a Representative cPLA2α protein expression in the spinal cord tissue sections along the hmSOD1 mouse life span. Scale bars = 250 μm for upper section and 50 μm for lower sections. The mean ± SEM of the cPLA2α fluorescence intensity is presented in the bar graph (n = 8 mice for each time point, five fields were analyzed for each mouse). *p < 0.001—significance from WT mice. b, c Development of the disease monitored by Kaplan-Meier survival curve and motor performance on accelerating rotarod test (n = 12 mice in each group). d Representative spinal cord sections of neurons (NeuN), microglia (Iba-1 and CD40), and astrocytes (GFAP) during the hmSOD1 mouse life span. The mean ± SEM of percentage of the cell area is presented in the bar graphs (n = 8 for each time point, five fields were analyzed for each mouse). Scale bars = 50 μm and in the inset 20 μm. *p < 0.001—significance from WT mice. e Representative staining of peripheral monocytes detected by double staining of Iba-1 and CD169, shown by arrows. Scale bars = 250 μm for left section and 50 μm for right section in each pair. Scale bars in the insets = 20 μm. The mean ± SEM of number of monocytes and of percentage of the cell area is presented in the bar graphs (n = 8 for each time point, five fields were analyzed for each mouse). *p < 0.001—significance in comparison to week 19
Elevated cPLA2α protein expression in the different cell types of the spinal cord. a Representative pictures of immunofluorescence double staining of cPLA2α (green) with motor neurons (NeuN), microglia (Iba-1), or astrocytes (GFAP) in the spinal cord during hmSOD1 mouse life span (out of 8 mice in each time point, presented in Fig. 1a). DAPI staining for cell nuclei. Scale bars = 20 μm. b Insets of the immunofluorescence double staining of cPLA2α with motor neurons, microglia, or astrocytes (presented in a), in a higher magnification. Scale bars = 20 μm. c Immunofluorescence double staining of phospho-cPLA2α (p-cPLA2) and motor neurons (NeuN), microglia (Iba-1) and astrocytes (GFAP). Shown representative immunofluorescence confocal microscopy pictures (out of 8 mice in each group). Lower panel presents the cell staining and DAPI to show the cell nuclei. Scale bars = 20 μm
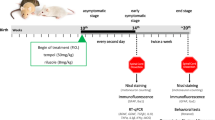
To determine whether cPLA2α has a role in the induction of the disease, its expression was blunted in the brain and spinal cord by means of specific oligonucleotide antisense against cPLA2α at 6-week-old hmSOD1 mice, when the elevation of cPLA2α was first detected. Ten micrograms per day of AS or the corresponding sense or saline was continuously pumped into the right lateral ventricle as done in our earlier study in a mouse model of amyloid beta brain infusion [19]. Using this methodology, it was shown [27] that significant oligonucleotide concentrations were achieved in the brain and brainstem and in all levels of the spinal cord. AS brain infusion to 6-week-old mice over a period of 6 weeks significantly prevented upregulation of cPLA2α in the brainstem as detected by immunofluorescence (Fig. 3a) and in the spinal cord as detected by Western blot analysis (Fig. 3b). As expected, at 12 weeks, there was no neuronal damage as well as no activation of microglia or astrocytes and the AS brain infusion had no effect (Fig. 3a). This treatment that did prevent the initial elevation of cPLA2α had no effect on the development of the disease assayed by motor function on rotarod (Fig. 3c). The initial value of 80 % of pre-symptomatic is due to the pump implantation surgery.
Reduction of cPLA2α upregulation at the early stage did not affect the development of the disease. Mice were brain infused with 10 μg/day AS (n = 12) or the corresponding sense (n = 6) or saline (n = 6) for 6 weeks starting at 6-week-old hmSOD1 mice. a Representative immunofluorescence staining of cPLA2α, neurons (NeuN), microglia (Iba-1), and astrocytes (GFAP) in the brain stem tissue section of WT mice, hmSOD1 mice (ALS), hmSOD1 mice brain infused with AS (ALS + AS) or sense (ALS + SE) at 12 weeks. Mice receiving saline or sense showed similar results and were combined under the sense-treated group. A double staining of cPLA2α and NeuN is presented. Scale bars = 50 μm, and in the insets = 20 μm. Shown are representative images of 12 mice in each group. The mean ± SEM of the fluorescence intensity (for cPLA2α) and percentage of the cell area (for cell markers) are presented in the bar graph. *p < 0.001—significance from WT mice and from AS-treated hmSOD1 mice. b A representative immunoblot analysis of cPLA2α and the corresponding calreticulin protein expression in the spinal cord lysates of the mice. cPLA2α protein expression was determined by dividing the intensity of each cPLA2α by the intensity of the corresponding calreticulin band after quantitation by densitometry and expressed in the bar graph as arbitrary units. The bar graph is the mean ± SE of 8 mice in each group. *p < 0.001—significance from WT mice and from AS-treated hmSOD1 mice. c Motor function by rotarod test of AS or sense brain infused-hmSOD1 mice (n = 12 in each group)
To determine the role of cPLA2α in the progression of the disease, 10 μg/day AS or sense was brain infused at week 15 (shortly before the onset of motor neuronal dysfunction) for 6 weeks (due to the pump’s limit). As shown in Fig. 4a, AS brain infusion prolonged the survival in mice by 12 days (<0.05). AS brain infusion significantly delayed the onset of motor neuron dysfunction by 3 weeks and there was a significant (p < 0.001) difference between the two groups until 21 weeks of age (Fig. 4b). Immediately after the pump implantation, there was a reduction in motor function, which was recovered only in the AS-treated hmSOD1 mice. The evaluation of the overall motor symptoms change in mice done by Ladder test (Fig. 4c) showed also a delay of 3 weeks in the loss of motor neuron function in the AS-treated hmSOD1 mice and a significant (p < 0.001) difference from the sense-treated hmSOD1 mice until 23 weeks of age.
AS brain infusion to hmSOD1 mice shortly before the onset of motor neuron dysfunction delayed development of the disease. Fifteen-week-old hmSOD1 mice were brain infused with 10 μg/day AS or the corresponding sense during 6 weeks (n = 12 in each group). AS brain infusion prolonged survival (a), delayed loss of motor function analyzed by Rotarod (b), and delayed neurological score analyzed by a ladder (c). *p < 0.001—significance from sense-treated hmSOD1 mice
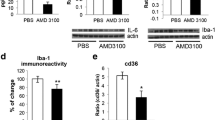
To determine the processes that are affected by cPLA2α during the development of the disease symptoms and to characterize the effect of prevention of cPLA2α upregulation on the different processes taking place at the first phase of development of motor neuron dysfunction, hmSOD1 mice were brain infused with AS or sense at week 15 for 3–4 weeks and the experiments were terminated for analysis at 18–19 weeks of age, at a time when the two mouse groups—AS and sense control—exhibited a significant difference in the disease symptoms. Non-treated hmSOD1 mice and WT mice at the same age were also studied. As shown in the immunofluorescence analysis and quantitation in Fig. 5a, cPLA2α protein expression was much lower in the spinal cord of 18–19-week-old AS-treated hmSOD1 mice in comparison with hmSOD1 mice or sense-treated hmSOD1 mice. AS treatment prevented the reduction in the number of the neurons (detected by NeuN), prevented astrocyte activation (detected by GFAP), and inhibited microglia activation (detected by Iba-1 and by CD40) compared with hmSOD1 mice or sense-treated hmSOD1 mice. In AS-treated hmSOD1 mice, reduction of cPLA2α upregulation led to diminution of the key inflammatory enzymes; iNOS and COX-2 as detected by Western blot analysis in the spinal cord lysates (Fig. 5b).
Reduction of cPLA2α upregulation in the spinal cord of hmSOD1 mice shortly before the onset of motor dysfunction reduced neuronal death and gliosis. Fifteen-week-old hmSOD1 mice were brain infused with 10 μg/day AS or the corresponding sense for 3–4 weeks and analyzed at week 18–19. a Representative immunofluorescence staining of cPLA2α, neurons (NeuN), microglia (Iba-1), and astrocytes (GFAP) in the spinal cord tissue section of WT mice, hmSOD1 mice (ALS), hmSOD1 mice brain infused with AS (ALS + AS) or sense (ALS + SE) at 18–19 weeks. Scale bars = 50 μm. Shown are representative images of 12 mice in each group. The mean ± SEM of the fluorescence intensity (for cPLA2α) and percentage of the cell area (for cell markers) are presented in the bar graph. *p < 0.001—significance from WT mice and from AS-treated hmSOD1 mice. b Representative immunoblot analysis of cPLA2α, COX-2, and iNOS and the corresponding calreticulin protein expression in the spinal cord lysates of the mice. Densitometry analysis for the three proteins was done as described for cPLA2α in Fig. 3b. The bar graph is the mean ± SE of 8 mice in each group. *p < 0.001—significance from WT mice and from AS-treated hmSOD1 mice
Discussion
We show herein that cPLA2α is upregulated in the spinal cord of 6-week-old mice long before the appearance of the disease symptoms, neuronal death, or gliosis and remained elevated during the whole life span of the hmSOD1 mice. Prevention of cPLA2α upregulation at the pre-symptomatic period (between 6 and 12 weeks) did not affect the course of the disease. However, prevention of cPLA2α upregulation shortly before the onset of the disease symptoms significantly (p < 0.001) delayed the loss of motor neuronal function, suggesting that cPLA2α upregulation in the spinal cord plays a role in the disease pathology. In line with our results, the role of elevated spinal cord cPLA2α in neuronal damage was reported also in spinal cord injury [28] and in spinal inflammatory hyperalgesia [29].
In the present study we used, specific antisense brain infusion in order to determine the specific role of cPLA2α upregulation in the spinal cord in the pathogenesis of ALS. The advantage of the AS technique over knockout mice is its ability to inhibit the induction of an accelerated protein at the site of inflammation, and enables its expression at basal levels, whereas in knockout mouse models total elimination of a protein may result in dramatic changes of homeostasis and/or compensation by upregulation of other proteins (with similar biological features). The use of AS strategy is reinforced by two recent studies in cPLA2α knockout mice reporting that total elimination of cPLA2α resulted with abnormalities in architecture of synapses and cortical neurons [30] as well as in alteration of brain phospholipid composition [31]. AS brain infusion to 15-week-old hmSOD1 mice (for 6 weeks) shortly before the development of motor dysfunction resulted in reduction of cPLA2α elevated protein expression (as detected at 18–19 weeks in the spinal cord) and significantly delayed the development of the disease symptoms. This blunting of cPLA2α upregulation resulted in inhibition of activation of microglia and astrocytes as well as inhibition of motor neuronal death, as detected by immunofluorescence analysis in the spinal cord of 18–19-week-old hmSOD1 mice.
A generation of mice expressing a conditional deletion of the mutant SOD1 gene showed that each individual cell types in the spinal cord contributes to the development of ALS [8, 32]. Likewise, replacing the myeloid lineage of mutant SOD1 mice with wild-type microglia slowed disease progression [6]. Since cPLA2α upregulation was detected in each cell type in the spinal cord, it probably contributes by different mechanisms to motor neuron dysfunction and cell death: (i) elevated neuronal cPLA2α can act directly in inducing neuronal death (ii) while elevated glial cPLA2α can act indirectly by activating microglia and/or astrocytes. In our recent study in primary neuronal cultures, we reported that cPLA2α activation and upregulation induced apoptotic neuronal death [18]. Concomitant with our results, cDNA microarray analysis to monitor gene expression in the spinal cord of hmSOD1 mice during neurodegeneration revealed changes in apoptosis-related gene expression [4]. In addition, the role of cPLA2α in glutamate excitotoxicity and oxidative stress-induced cell death in primary spinal cord neuron cultures was recently reported [33]. Taken together the studies in neuronal cultures and the abundant levels of cPLA2α in motor neurons in the spinal cord, it is suggested that neuronal cPLA2α plays a direct role in motor neuron cell death. Similar to other neurodegenerative diseases and acute CNS insults, one of the most striking hallmarks of ALS shared by familial and sporadic patients, as well as by rodent models, is neuro-inflammation, characterized by extensive microglial activation and astrogliosis that contribute to disease progression, rather than to its resolution [2, 34–36]. Reduction of cPLA2α upregulation in the spinal cord of the hmSOD1 mice prevented astrocyte activation detected by GFAP immunostaining that is in line with in vitro studies [37] reporting that activation of cPLA2α led to an increase in oxidative stress in astrocytes. We show here a massive activation of microglia in the spinal cord (detected by immunostaining of Iba-1 and CD40) that preceded the changes in the motor neurons, in accordance with other studies [6, 38, 39]. This microglia activation was shown to be cPLA2α dependent coincided with ours and others’ studies in cell cultures demonstrating the specific role of microglial cPLA2α in the activation and transformation of microglia to M1 phenotype. We have previously reported that cPLA2α activity regulated the production of superoxides by NOX-2 NADPH oxidase and the induction of COX-2 and iNOS via nuclear factor kB (NF-kB) in microglia cultures [17]. Interestingly, microglial NF-kB specifically has been shown to play a major role in the development of the ALS in hmSOD1 mice [40]. We show here that reduction of cPLA2α in the spinal cord also decreased iNOS and COX-2 upregulation that produce two major proinflammatory mediators; nitric oxide and PGE2, respectively. As shown in the present study for cPLA2α, it was also reported that in both early symptomatic and end-stage transgenic hmSOD1 mice, neurons and to a lesser extent glial cells in the spinal cord exhibit robust COX-2 [41] and iNOS immunoreactivity [42]. Likewise, similar to cPLA2α, COX-2 was dramatically increased in postmortem spinal cord samples from sporadic ALS patients [ 41]. Nitric oxide and superoxides both under cPLA2α regulation [17] can form the toxic reagent peroxynitrite [43, 44]. In this context, a recent study reported that inhibition of cPLA2α activity ameliorated experimental autoimmune encephalomyelitis via blocking peroxynitrite formation in mouse spinal cord white matter [45]. These results suggest that glial cPLA2α is involved by different inflammatory processes in the development of ALS. In accordance with this suggestion, injections of PLA2 into the spinal cord of mice caused inflammation and oxidative stress [46].
The cause of cPLA2α elevation in the brain and spinal cord as early as 6-week-old mice is not yet clear. SOD1 insoluble protein complexes (IPCs) were detected in motor neurons of 30-day-old mSOD1 mice [47], before the manifestation of ALS pathology, and several months before the appearance of inclusion bodies. It may be possible that the formations of IPCs in the neurons triggered the elevation of cPLA2α. The role of this early elevation of cPLA2α is also not known and prevention of this elevation for 6 weeks had no effect on the course of the disease.
The role of monocyte recruitment to the spinal cord in ALS is under debate. On one hand, it was reported that inhibition of monocyte recruitment to the spinal cord delayed motor dysfunction and increased the survival of ALS mice [48]. In contrast, another study [49] reported that monocytes could not be detected in the spinal cord of SOD1G93A mice and that microglia are not derived from infiltrating monocytes. Likewise, a recent study [50] reported that in the mutant SOD1G93A mouse model of ALS, the choroid plexus of the brain did not support leukocyte trafficking during disease progression, due to a local reduction in IFN-γ levels. The results of the present study, in agreement with the two later reports, show that the recruitment of monocytes detected by co-immunostaining with CD169 and Iba-1 was evident only at the end stage.
Conclusions
We show here that cPLA2α is elevated at 6 weeks, long before the appearance of the disease symptoms. cPLA2α elevation at the onset of symptoms plays a role in the induction of motor dysfunction. Reduction of cPLA2α upregulation shortly before the loss of motor neurons function significantly delayed the appearance of the disease symptoms. Elevated neuronal and glial cPLA2α may contribute by different aspects involved in the pathogenesis of ALS. Thus, cPLA2α may offer an efficient target for treatment of ALS and potentially other multifactorial neurodegenerative diseases.
Abbreviations
ALS, amyotrophic lateral sclerosis; AS, antisense oligonucleotide against cPLA2α; C9ORF72, chromosome 9 open reading frame 72; COX-2, cyclooxygenase-2; cPLA2α, cytosolic phospholipase A2 alpha; hmSOD1, human mutant SOD1G93A; iNOS, inducible nitric oxide synthase; NF-kB, nuclear factor kB; SOD1, superoxide dismutase; TDP-43, TAR DNA binding protein
References
Ince P, Highley J, Kirby J, Wharton S, Takahashi H, Strong M, Shaw P. Molecular pathology and genetic advances in amyotrophic lateral sclerosis: an emerging molecular pathway and the significance of glial pathology. Acta Neuropathol. 2011;122:657–71.
Andersen P. Genetics of sporadic ALS. Amyotroph Lateral Scler Other Motor Neuron Disord. 2001;2:S37–41.
Philips T, Robberecht W. Neuroinflammation in amyotrophic lateral sclerosis: role of glial activation in motor neuron disease. Lancet Neurol. 2011;10:253–63.
Yoshihara T, Ishigaki S, Yamamoto M, Liang Y, Niwa J, Takeuchi H, Doyu M, Sobue G. Differential expression of inflammation- and apoptosis-related genes in spinal cords of a mutant SOD1 transgenic mouse model of familial amyotrophic lateral sclerosis. J Neurochem. 2002;80:158–67.
Chiu I, Phatnani H, Kuligowski M, Tapia J, Carrasco M, Zhang M, Maniatis T, Carroll M. Activation of innate and humoral immunity in the peripheral nervous system of ALS transgenic mice. Proc Natl Acad Sci U S A. 2009;106:20960–5.
Beers D, Henkel J, **ao Q, Zhao W, Wang J, Yen A, Siklos L, McKercher S, Appel S. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2006;103:16021–6.
Nagai M, Re D, Nagata T, Chalazonitis A, Jessell T, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–22.
Boillee S, Yamanaka K, Lobsiger C, Copeland N, Jenkins N, Kassiotis G, Kollias G, Cleveland D. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–92.
Chiu I, Chen A, Zheng Y, Kosaras B, Tsiftsoglou S, Vartanian T, Brown RJ, Carroll M. T lymphocytes potentiate endogenous neuroprotective inflammation in a mouse model of ALS. Proc Natl Acad Sci U S A. 2008;105:17913–8.
Kramer RM, Roberts EF, Manetta J, Putnam JE. The Ca2(+)-sensitive cytosolic phospholipase A2 is a 100-kDa protein in human monoblast U937 cells. J-Biol-Chem. 1991;266:5268–72.
Clark JD, Milona N, Knopf JL. Purification of a 110-kilodalton cytosolic phospholipase A2 from the human monocytic cell line U937. Proc Natl Acad Sci U S A. 1990;87:7708–12.
Stephenson DT, Lemere CA, Selkoe DJ, Clemens JA. Cytosolic phospholipase A2 (cPLA2) immunoreactivity is elevated in Alzheimer’s disease brain. Neurobiol Dis. 1996;3:51–63.
Clemens JA et al. Reactive glia express cytosolic phospholipase A2 after transient global forebrain ischemia in the rat. Stroke. 1996;27:527–35.
Stephenson D, Rash K, Smalstig B, Roberts E, Johnstone E, Sharp J, Panetta J, Little S, Kramer R, Clemens J. Cytosolic phospholipase A2 is induced in reactive glia following different forms of neurodegeneration. Glia. 1999;27:110–28.
Shibata N, Kakita A, Takahashi H, Ihara Y, Nobukuni K, Fujimura H, Sakoda S, Kobayashi M. Increased expression and activation of cytosolic phospholipase A2 in the spinal cord of patients with sporadic amyotrophic lateral sclerosis. Acta Neuropathol. 2010;119:345–54.
Kiaei M, Kipiani K, Petri S, Choi D, Chen J, Calingasan N, Beal M. Integrative role of cPLA with COX-2 and the effect of non-steroidal anti-inflammatory drugs in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurochem. 2005;93:403–11.
Szaingurten-Solodkin I, Hadad N, Levy R. Regulatory role of cytosolic phospholipase A2alpha in NADPH oxidase activity and in inducible nitric oxide synthase induction by aggregated Abeta1-42 in microglia. Glia. 2009;57:1727–40.
Sagy-Bross C, Hadad N, Levy R. Cytosolic phospholipase Aalpha upregulation mediates apoptotic neuronal death induced by aggregated amyloid-beta peptide. Neurochem International. 2013;63:541–50.
Sagy-Bross C, Kasianov K, Solomonov Y, Braiman A, Friedman A, Hadad N, Levy R. The role of cytosolic phospholipase A alpha in amyloid precursor protein induction by amyloid beta : implication for neurodegeneration. J Neurochem. 2015;132:559–71.
Craft JM, Watterson DM, Van Eldik LJ. Human amyloid beta-induced neuroinflammation is an early event in neurodegeneration. Glia. 2006;53:484–90.
Craft JM, Van Eldik LJ, Zasadzki M, Hu W, Watterson DM. Aminopyridazines attenuate hippocampus-dependent behavioral deficits induced by human beta-amyloid in a murine model of neuroinflammation. J Mol Neurosci. 2004;24:115–22.
Craft JM, Watterson DM, Frautschy SA, Van Eldik LJ. Aminopyridazines inhibit beta-amyloid-induced glial activation and neuronal damage in vivo. Neurobiol Aging. 2004;25:1283–92.
Raichel L, Berger S, Hadad N, Kachko L, Karter M, Szaingurten-Solodkin I, Levy R. Reduction of cPLA2alpha overexpression: an efficient anti-inflammatory therapy for collagen-induced arthritis. Eur J Immunol. 2008;38:2905–15.
Baron R, Babcock AA, Nemirovsky A, Finsen B, Monsonego A. Accelerated microglial pathology is associated with Abeta plaques in mouse models of Alzheimer’s disease. Aging Cell. 2014;13:584–95.
Weissberg I, Wood L, Kamintsky L, Vazquez O, Milikovsky DZ, Alexander A, Oppenheim H, Ardizzone A, Becker A, Frigerio F. Albumin induces excitatory synaptogenesis through astrocytic TGF-beta/ALK5 signaling in a model of acquired epilepsy following blood-brain barrier dysfunction. Neurobiol Dis. 2015;78:115–25.
Benkler C, Barhum Y, Ben-Zur T, Offen D. Multifactorial gene therapy enhancing the glutamate uptake system and reducing oxidative stress delays symptom onset and prolongs survival in the SOD1-G93A ALS mouse model. J Mol Neurosci. 2016;58:46–58.
Smith R, Miller T, Yamanaka K, Monia B, Condon T, Hung G, Lobsiger C, Ward C, McAlonis-Downes M, Wei H. Antisense oligonucleotide therapy for neurodegenerative disease. J Clin Invest. 2006;116:2290–6.
Liu NK, Deng LX, Zhang YP, Lu QB, Wang XF, Hu JG, Oakes E, Bonventre JV, Shields CB, Xu XM. Cytosolic phospholipase A2 protein as a novel therapeutic target for spinal cord injury. Ann Neurol. 2014;75:644–58.
Lucas KK, Svensson CI, Hua XY, Yaksh TL, Dennis EA. Spinal phospholipase A2 in inflammatory hyperalgesia: role of group IVA cPLA2. Br J Pharmacol. 2005;144:940–52.
Qu BX, Gong Y, Sinclair D, Fu M, Perl D, Diaz-Arrastia R. cPLA2alpha knockout mice exhibit abnormalities in the architecture and synapses of cortical neurons. Brain Res. 2013;1497:101–5.
Rosenberger TA, Villacreses NE, Contreras MA, Bonventre JV, Rapoport SI. Brain lipid metabolism in the cPLA2 knockout mouse. J Lipid Res. 2003;44:109–17.
Yamanaka K, Boillee S, Roberts EA, Garcia ML, McAlonis-Downes M, Mikse OR, Cleveland DW, Goldstein LS. Mutant SOD1 in cell types other than motor neurons and oligodendrocytes accelerates onset of disease in ALS mice. Proc Natl Acad Sci U S A. 2008;105:7594–9.
Zhao Z, Liu N, Huang J, Lu PH, Xu XM. Inhibition of cPLA2 activation by Ginkgo biloba extract protects spinal cord neurons from glutamate excitotoxicity and oxidative stress-induced cell death. J Neurochem. 2011;116:1057–65.
Nguyen MD, Julien JP, Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci. 2002;3:216–27.
**ao Q, Zhao W, Beers DR, Yen AA, **e W, Henkel JS, Appel SH. Mutant SOD1(G93A) microglia are more neurotoxic relative to wild-type microglia. J Neurochem. 2007;102:2008–19.
Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–94.
Zhu D, Lai Y, Shelat PB, Hu C, Sun GY, Lee JC. Phospholipases A2 mediate amyloid-beta peptide-induced mitochondrial dysfunction. J Neurosci. 2006;26:11111–9.
Hall ED, Oostveen JA, Gurney ME. Relationship of microglial and astrocytic activation to disease onset and progression in a transgenic model of familial ALS. Glia. 1998;23:249–56.
Alexianu ME, Kozovska M, Appel SH. Immune reactivity in a mouse model of familial ALS correlates with disease progression. Neurology. 2001;57:1282–9.
Frakes AE, Ferraiuolo L, Haidet-Phillips AM, Schmelzer L, Braun L, Miranda CJ, Ladner KJ, Bevan AK, Foust KD, Godbout JP. Microglia induce motor neuron death via the classical NF-kappaB pathway in amyotrophic lateral sclerosis. Neuron. 2014;81:1009–23.
Almer G, Guegan C, Teismann P, Naini A, Rosoklija G, Hays AP, Chen C, Przedborski S. Increased expression of the pro-inflammatory enzyme cyclooxygenase-2 in amyotrophic lateral sclerosis. Ann Neurol. 2001;49:176–85.
Almer G, Vukosavic S, Romero N, Przedborski S. Inducible nitric oxide synthase up-regulation in a transgenic mouse model of familial amyotrophic lateral sclerosis. J Neurochem. 1999;72:2415–25.
Bal-Price A, Matthias A, Brown G. Stimulation of the NADPH oxidase in activated rat microglia removes nitric oxide but induces peroxynitrite production. J Neurochem. 2002;80:73–80.
Li J, Baud O, Vartanian T, Volpe J, Rosenberg P. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc Natl Acad Sci U S A. 2005;102:9936–41.
Vana A, Li S, Ribeiro R, Tchantchou F, Zhang Y. Arachidonyl trifluoromethyl ketone ameliorates experimental autoimmune encephalomyelitis via blocking peroxynitrite formation in mouse spinal cord white matter. Exp Neurol. 2011;231:45–55.
Liu NK, Zhang YP, Titsworth WL, Jiang X, Han S, Lu PH, Shields CB, Xu XM. A novel role of phospholipase A2 in mediating spinal cord secondary injury. Ann Neurol. 2006;59:606–19.
Johnston JA, Dalton MJ, Gurney ME, Kopito RR. Formation of high molecular weight complexes of mutant Cu, Zn-superoxide dismutase in a mouse model for familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2000;97:12571–6.
Butovsky O, Siddiqui S, Gabriely G, Lanser A, Dake B, Murugaiyan G, Doykan C, Wu P, Gali R, Iyer L. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J Clin Invest. 2012;122:3063–87.
Chiu I, Morimoto E, Goodarzi H, Liao J, O'Keeffe S, Phatnani H, Muratet M, Carroll M, Levy S, Tavazoie S. A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep. 2013;4:385–401.
Kunis G, Baruch K, Miller O, Schwartz M. Immunization with a myelin-derived antigen activates the brain’s choroid plexus for recruitment of immunoregulatory cells to the CNS and attenuates disease progression in a mouse model of ALS. J Neurosci. 2015;35:6381–93.
Acknowledgements
We thank Dr. D. Offen from Felsenstein Medical Research Center, Sackler School of Medicine, Tel Aviv University, Tel Aviv and Rabin Medical Center, Petah Tikva, Israel, for teaching us the method for Evaluating Neurological Score by Ladder Testing.
This research was supported by a grant from the Israel Sciences Foundation founded by the Israel Academy of Sciences and Humanities 1012/09.
Availability of data and materials
Information about the animal model, experimental methods, and data described in this paper are available to scientific and medical communities for review, verification, and research studies.
Authors’ contributions
YS designed and carried out all the experiments, researched the data, prepared the figures, and participated in writing the manuscript. NH participated in the design of the study and guided some methodologies. RL designed the study, directed the study, and wrote the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicative.
Ethics approval and consent to participate
The study was approved by the Ben-Gurion University Institutional Animal Care and Use Committee (IL-37-05-2012) and was conducted according to the Israeli Animal Welfare Act following the guidelines of the Guide for Care and Use of Laboratory Animal (National Research Council, 1996).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Solomonov, Y., Hadad, N. & Levy, R. Reduction of cytosolic phospholipase A2α upregulation delays the onset of symptoms in SOD1G93A mouse model of amyotrophic lateral sclerosis. J Neuroinflammation 13, 134 (2016). https://doi.org/10.1186/s12974-016-0602-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12974-016-0602-y