Abstract
Glioblastoma multiforme (GBM) is the most common malignant brain cancer that invades normal brain tissue and impedes surgical eradication, resulting in early local recurrence and high mortality. In addition, most therapeutic agents lack permeability across the blood brain barrier (BBB), further reducing the efficacy of chemotherapy. Thus, effective treatment against GBM requires tumor specific targets and efficient intracranial drug delivery. With the most recent advances in immunotherapy, genetically engineered T cells with chimeric antigen receptors (CARs) are becoming a promising approach for treating cancer. By transducing T lymphocytes with CAR constructs containing a tumor-associated antigen (TAA) recognition domain linked to the constant regions of a signaling T cell receptor, CAR T cells may recognize a predefined TAA with high specificity in a non-MHC restricted manner, and is independent of antigen processing. Active T cells can travel across the BBB, providing additional advantage for drug delivery and tumor targeting. Here we review the CAR design and technical innovations, the major targets that are in pre-clinical and clinical development with a focus on GBM, and multiple strategies developed to improve CAR T cell efficacy.
Similar content being viewed by others
Background
Glioblastoma (GBM), WHO grade IV glioma, is the most devastating brain tumor in adults [55]. O’Rourke et al. [41] reported the first study in humans of intravenous delivery of a single dose of EGFRvIII-CAR T cells in 10 recurrent GBM patients which showed safety and limited anti-tumor response as well as targeted antigen downregulation. Goff et al. [56] reported a pilot phase I trial with third generation EGFRvIII-CAR T cells administered after lymphodepleting chemotherapy and intravenous interleukin-2 injections. This study did not show significant toxicity but failed to show clinical efficacy. Another design of EGFRvIII-CAR T cell using humanized scFv again proved safe but also failed to show clinical benefit [41, 57]. Currently, there are six EGFRvIII-CAR T cell clinical trials ongoing with two in combination with chemotherapy or ICI (Table 1).
HER2
HER2 is a member of the EGFR family, also named as ERBB2. Increased levels of HER2 protein in GBM patients is linked to poor survival [14, 58, 59]. Unlike EGFRvIII, which is unique to GBM, HER2 is overexpressed in many cancer types including breast, ovarian and GBM; it also is expressed in some normal tissues, leading to safety concerns. An early case report with HER2-CAR T cell therapy has documented a metastatic colon cancer patient who experienced respiratory distress within 15 min of HER2-targeting CAR T cell administration and died 5 days after treatment. Serum samples after cell infusion showed marked increases in interferon γ (IFNγ), granulocyte macrophage-colony stimulating factor (GM-CSF), tumor necrosis factor-alpha (TNF-alpha), interleukin-6 and -10 (IL-6 and IL-10), consistent with a cytokine storm [60]. Since then, efforts have been made to overcome these safety concerns [61]. Liu et al. [62] showed that lowering the binding affinity between scFv and HER2 may increase the differential binding of CAR T cells to tumor versus normal tissue in preclinical cancer models, providing a good strategy for targeting the antigens that are not specific to solid tumors. A more recent clinical trial (NCT00902044) reported no significant toxicities of a second generation HER2-specific CAR T-cell therapy for sarcoma [63]. Compared with the original 4D5 HER2-CAR which binds to the juxtamembrane region of HER2 protein and caused severe toxicity, this trial used a FRP5-HER2 CAR which recognizes a discontinuous epitope between residues 11–169 of HER2 that is more distant from the cell surface. Thus, the binding site of the epitope also determines the activity of the CARs. As for GBM, investigators at Baylor College of Medicine have conducted a clinical trial (NCT01109095) to evaluate the safety and efficacy of HER2-specific CARs using virus-specific T cells (CAR-VSTs) [59]; previous studies have shown that virus engineered cytotoxic T cells demonstrate better persistence and T cell expansion through appropriate co-stimulatory signaling activation [64]. In this trial, overexpression of FRP5 HER2-CARs in virus-specific T cells not only improved the specific targeting of HER2+ tumor cells, but also prolonged the HER2-CAR VST cell persistence and antitumor activity. While this trial has demonstrated treatment safety, the clinical benefit was limited.
EphA2
EphA2 protein is overexpressed in gliomas and is associated with malignancy, thus it became a good molecular target in GBM [65, 66]. Preclinical studies demonstrated that a second generation EphA2-CAR T cell induced glioma xenograft tumor regression in vivo [46, 67]. So far one clinical trial was initiated to evaluate the safety and effectiveness of CAR T cell immunotherapy in treating patients with EphA2+ malignant glioma but has been withdrawn recently for unknown reason (NCT02575261).
GD2
GD2 is a glycosphingolipid expressed at low levels on the surface of healthy cells, but highly expressed on several tumor types including gliomas and is associated with increased tumor proliferation and invasion [68]. Pre-clinical studies have demonstrated a robust antigen-dependent cytokine production and killing of GD2+-positive glioma cells in vitro and in vivo using patient-derived orthotopic xenograft models overexpressing GD2 [16, 17]. Currently, two phase I clinical trials (NCT04099797 and NCT03423992) are evaluating the safety and efficacy of GD2-specific CAR T cells in high grade glioma and diffuse intrinsic pontine glioma (DIPG).
B7-H3
B7-H3 (CD276) is an immune checkpoint molecule and a member of B7 protein superfamily. B7-H3 binds to the majority of neuroepithelial tumors but not to normal glia or tissues, making it a promising target for therapeutics [69]. Clinical trials for two mAbs targeting B7-H3 (8H9 and MGA271) have been shown to be safe and promising for metastatic CNS neuroblastoma and DIPG in children.[70, 71]. Using a B7-H3 (MGA271) 41BBζ CAR, Majzner et al. [18] have reported a robust anti-tumor activity in multiple solid, liquid, and CNS tumor types. In addition, Nguyen et al. [72] demonstrated that B7-H3-specific (MGA271) CD28ζ CAR-T cells have potent anti-tumor activity in U373 glioma model. To date, two GBM-related clinical trials focused on B7-H3 CAR T-cell therapy (NCT04385173, NCT04077866) are in the recruitment phase.
Chlorotoxin
Chlorotoxin (CLTX) is a 36-amino acid peptide first isolated from scorpion venom that specifically binds to GBM but not to normal tissue. Pharmacologically, CLTX binds to and blocks small-conductance chloride channels [73]. Unlike the common CARs that are designed to recognize surface TAAs to kill tumor cells, the CLTX-CAR was designed as a peptide-based CAR to recapitulate the GBM-binding potential of CLTX. With this approach, Wang et al. [19] showed that CLTX-CAR T cells mediate potent anti-GBM activity and efficiently targeted tumors lacking expression of other GBM-associated antigens, resulting in tumor regression in orthotopic xenograft GBM tumor models. Importantly, the CLTX-CAR-T cells exhibited minimal off-target effects, without showing toxicity following systemic or regional delivery into mice. Given the finding that effective targeting by CLTX-CAR T cells requires cell surface expression of matrix metalloproteinase-2 (MMP2), a clinical trial has been initiated for treating MMP2+ recurrent GBM (NCT04214392). More importantly, this study also opened a new avenue to repurpose the use of a natural toxin for CAR T cell engineering.
Other potential CAR T cell targets for GBM
While the current CAR T cell targets continue to be developed and improved, progress also is being made in exploring new targets specific for GBM. Below we review other promising brain TAAs that have been tested pre-clinically.
CD70
CD70 is a type II transmembrane protein and a member of the tumor necrosis factor family. CD70 was not detected in normal peripheral and brain tissues but was constitutively overexpressed in isocitrate dehydrogenase (IDH) wild-type primary low-grade gliomas, and GBMs in the mesenchymal subgroup and recurrent tumors. CD70 is associated with poor survival in GBM patients making it a good candidate for CAR T-cell therapy [74, 75]. Several pre-clinical studies with differently designed CD70-specific CAR-T cells have shown robust anti-tumor response against CD70+ mouse models [74, 76]. While there is no current clinical trial for testing CD70-specific CAR-T cells in GBM, phase I/II clinical trials are underway to study the safety and efficacy of CD70-specific CAR-T cell therapy in B cell cancers (NCT03125577, NCT04429438) as well as pancreatic, renal, breast and ovarian cancers (NCT02830724).
CD133
CD133 is a marker for self-renewing cancer stem cells (CSCs) in solid tumors. CD133+ tumor cells, including GBM, are known to be highly resistant to chemo- and radiotherapy. Recently, CD133 has been identified as a potential CAR T cell target for treatment of GBM. While CD133 also is expressed on hematopoietic stem and progenitor cells (HSPC), Vora et al. [77] demonstrated that intra-tumoral injections of CD133-specific CAR T cells are effective at eliminating GBM and that this treatment does not cause systemic toxicity in humanized mouse models. In addition, in a phase 1 clinical trial (NTC 02541370) CD133-specific CAR T cells showed feasibility and efficacy against advanced metastatic malignancies with no serious adverse effects, further confirming the therapeutic potential of targeting CD133 [78].
MET
MET is the receptor of hepatocyte growth factor (HGF), and a well-known RTK being developed as an anti-cancer target. HGF/MET overexpression frequently occur in GBM patients and is associated with poor prognosis [54, 79, 80]. Using a transgenic mouse model, Qin et al. [81] showed that overexpression of HGF and MET may transform neuro stem cells into glioma stem cells (GSCs), leading to GBM initiation. GBM harboring MET amplification or HGF autocrine activation are sensitive to MET inhibitors in preclinical models [82, 83], recent clinical trials further showed that a combination of MET and VEGF inhibitors (ontarzucimab plus bevacizumab vs. placebo plus bevacizumab) significantly improved progression free survival (PFS) and overall survival (OS) in the mesenchymal subtype of recurrent GBM patients with high tumor HGF expression [84]. These results suggest that MET maybe a good target for CAR T-cell therapy in GBM.
Summary for CAR T cell targets in GBM
Although a large number of CAR T cell targets under development for treating GBM are showing promising preclinical results, limited antitumor response has been observed in clinical trials, largely due to limited T cell persistence and antigen-negative relapses. While technical innovations to improve the CAR T cell expansion, survival, efficacy and safety remain the key next steps to pursue, combination with other therapies also have been comprehensively considered to improve the CAR T cell therapy [85]. Below, we introduce the combination with ICIs as a promising approach to overcome T cell exhaustion which in turn may improve CAR T cell efficacy in clinical trials.
CAR T cell therapy in combination with immune checkpoint inhibitors
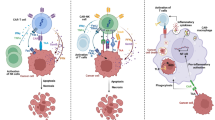
The tumor microenvironment is orchestrated by immunosuppressive cytokines, regulatory modulators and co-inhibitory receptors that regulate the responsiveness of immune cells [86]. While active CAR T cells elicit specific recognition and killing activities against tumor cells, the chronic exposure to tumor cells results in T cell exhaustion mediated by immune checkpoint pathway activation that is initiated by tumor cells, leading to a reduced ability to proliferate, produce cytokines and attack the tumor (Fig. 1c). Exhausted T cells upregulate immune inhibitory receptors, such as programmed death ligand-1 (PD-L1) and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4). Blocking this immune suppressive signaling has led to the development of ICIs such as nivolumab and durvalumab (PD-1/PD-L1 inhibitors) and ipilimumab (CTLA-4 inhibitors). These ICIs are FDA-approved for treating several types of cancer including melanoma, hepatocellular carcinoma and lymphoma and have shown significant clinical results [87]. Since ICIs eliminate T cell exhaustion, combining their use may further enhance CAR T-cell therapy efficacy through extended T cell proliferation and sustained tumor-killing activity. A recent case report has shown that administration of the PD-1/PD-L1 inhibitor pembrolizumab after CD19-specific CAR T-cell therapy in refractory diffuse large B-cell lymphoma induced a clinically significant antitumor response, suggesting that the PD-1 pathway may be critical in determining the response to CAR-modified T-cell immunotherapy [88]. However, despite the promising clinical results in other types of cancer, the use of ICIs in GBM clinical trials remain controversial. Nivolumab, a PD-1/PD-L1 inhibitor, failed to prolong overall survival of patients with recurrent GBM, leading to a discussion of whether ICIs may benefit GBM patients after all [89,90,91]. Nevertheless, a recent trial with 35 patients with recurrent, surgically-removable GBM showed that patients who receive neoadjuvant PD-1 blockade, with continued adjuvant therapy following surgery had significantly better overall survival compared to those who received adjuvant or post-surgical PD-1 blockade alone [92]. Another study also suggests that combination of local chemotherapy with PD-1 blockade enhanced antigen-specific T effector cell expansion and improves survival in GBM models [93]. These studies suggest that anti-PD-1 blockade alone may not directly benefit GBM patients but may improve the efficacy of other therapeutics when used in combination. Currently, clinical trials are on-going to evaluate the therapeutic efficacy of EGFRvIII-CAR (NCT03726515) and IL13Rα2-CAR (NCT04003649) T-cell therapy in combination with ICIs such as pembrolizumab or nivolumab in recurrent or refractory GBM patients (Table 1). We anticipate that anti-PD1 blockage may improve the CAR T-cell efficacy for treating GBM patients.
Future perspectives
Over the past decades, we have learned key principles to manage CAR T-cell efficacy: the specificity of the targeted antigen, the sufficient TCR activation by the specific antigen binding domain, the level of T cell activation and longevity, and the hostile micro-environment for T cells to penetrate, which also serves a source of immunosuppressive factors [94]. While many types of CAR T cells are now in clinical trials (Table 1), further optimization with advanced approaches are expected to improve their overall clinical efficacy.
Since genetic modification of CAR vectors is showing promising results in improving CAR T-cell efficacy, gene editing of the T cell is now a new strategy to further improve CAR T cells. Recently, the CRISPR/Cas9 mediated gene editing system has been applied to delete or mutate checkpoint-associated genes in CAR T cells, and has shown improvement in T cell persistence and survival [95,96,97]. Using CRISPR/Cas 9-mediated deletion, Ren et al. [95] generated CD19-CAR T cells simultaneously deficient in TCR, HLA class I molecule and PD1, which showed more potent antitumor activities than non-edited CAR T cells when tested in xenograft mouse models. Notably, the TCR and HLA class I double-deficient T cells further reduced MHC restriction and eliminated alloreactivity and potential graft-versus-host disease (GVHD), thus making the strategy particularly useful for treating different patients. A different study using CRISPR/Cas9-mediated deletion of TCR α subunit constant (TRAC) region, beta-2 microglobulin (B2M) and PD-1 also showed profound anti-tumor activity in vivo [97]. Recently, Li et al. [98] investigated the approaches to improve CAR T transduction efficacy and CRISPR/Cas9 mediated PD-1 deletion using a 2-in-1 lentivirus vector and found that blocking anti-viral signaling in human primary T cells enhanced lentiviral-mediated transduction efficacy of a combinatory CAR/CRISPR vector for targeting HER2 and inhibiting PD-1 at the same time. Most importantly, the first phase 1 clinical trial using CRISPR/Cas9 for multiplex gene editing on T cells (NCT03399448) has demonstrated safety and feasibility in three patients with refractory cancer [99]. Given that the CRISPR/Cas9 approach has been widely applied to functional gene screening and validation, it also opens a new avenue to discover and target novel modulators of immune suppression. Through CRISPR/Cas9-mutagenesis screening, Wei et al. [100] have identified Regnase-1 as a major negative regulator of anti-tumor responses in CD8+ T cells, and that CD19-CAR T cells with deleted Regnase-1 showed greater longevity and a more vigorous anti-tumor response in B16 ovarian and B6 melanoma mouse models. We anticipate that future GBM-specific studies also will explore these novel ideas and approaches, which hopefully will further enhance CAR T-cell proliferation, longevity and long-term anti-tumor activity.
Conclusion
Brain tumors are considered one of the “most-difficult-to- treat” solid tumors. With the most recent advances in immunotherapy, the CAR T-cell therapy has become a revolutionary approach for treating hematological malignancies and it has great potential for brain tumors. This review intends to introduce interpretations of the most relevant papers addressing the use of CAR T cells for the treatment of GBM. We discussed the CAR designs and optimization, the major CAR T cell targets in clinical trials, as well as the strategies developed to improve CAR-T cell efficacy in the context of GBM. We anticipate that future clinical trial designs will not only focus on efficacy and safety, but also on the mechanisms involved in the immune response and resistance, and that the next generation of CAR T cell therapy will become an effective and safe therapeutics for treating malignant GBM.
Availability of data and materials
All data are available in the manuscript or upon request to the authors.
Abbreviations
- ALL:
-
Acute lymphoblastic leukemia
- ACT:
-
Adoptive cell transfer
- BBB:
-
Blood brain barrier
- B2M:
-
Beta-2 microglobulin
- CSC:
-
Cancer stem cells
- CAR:
-
Chimeric antigen receptors
- CRISPR:
-
Clustered regularly interspaced short palindromic repeats
- Cas9:
-
CRISPR-associated protein 9
- CRS:
-
Cytokine release syndrome
- CTLA-4:
-
Cytotoxic T lymphocyte-associated antigen 4
- DIPG:
-
Diffuse intrinsic pontine glioma
- EGFRvIII:
-
Epidermal growth factor receptor variant III
- EphA2:
-
Erythropoietin-producing hepatocellular carcinoma A2
- GBM:
-
Glioblastoma multiforme
- GVHD:
-
Graft-versus-host disease
- GD2:
-
Ganglioside 2
- Frα:
-
Folate receptor α
- HGF:
-
Hepatocyte growth factor
- HER2:
-
Human epidermal growth factor 2
- IL13Rα2:
-
Interleukin-13 receptor alpha 2
- HSPC:
-
Hematopoietic stem and progenitor cells
- IDH:
-
Isocitrate dehydrogenase
- GM-CSF:
-
Granulocyte macrophage-colony stimulating factor
- TNF-alpha:
-
Tumor necrosis factor-alpha
- IL:
-
Interleukin
- IFNγ:
-
Interferon γ
- MHC:
-
Major histocompatibility complex
- MMP2:
-
Matrix metalloproteinase-2
- OS:
-
Overall survival
- PD-1:
-
Programmed death-1
- PD-L1:
-
Programmed death ligand-1
- PFS:
-
Progression free survival
- scFv:
-
Single chain variable domain of monoclonal antibodies
- SUPRA CAR:
-
Split, universal and programmable CAR
- TAA:
-
Tumor associated antigen
- TCR:
-
T cell receptors
- TRAC:
-
TCRα subunit constant
- TRUCKs:
-
T cells redirected for universal cytokine-mediated killing
- uCAR:
-
Universal CAR
References
**e Q, Mittal S, Berens ME. Targeting adaptive glioblastoma: an overview of proliferation and invasion. Neuro-oncology. 2014;16(12):1575–84.
Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319(25):1676–80.
Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;86(15):1159–66.
Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298(5594):850–4.
Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23(10):2346–57.
Sadelain M. CAR therapy: the CD19 paradigm. J Clin Invest. 2015;125(9):3392–400.
June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361–5.
O’Leary MC, Lu X, Huang Y, Lin X, Mahmood I, Przepiorka D, Gavin D, Lee S, Liu K, George B, et al. FDA approval summary: tisagenlecleucel for treatment of patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Clin Cancer Res. 2019;25(4):1142–6.
Vaquero J, Martinez R, Oya S, Coca S, Barbolla L, Ramiro J, Salazar FG. Intratumoural injection of autologous lymphocytes plus human lymphoblastoid interferon for the treatment of glioblastoma. Acta Neurochir. 1989;98(1–2):35–41.
Steinbok P, Thomas JP, Grossman L, Dolman CL. Intratumoral autologous mononuclear cells in the treatment of recurrent glioblastoma multiforme. A phase 1 (toxicity) study. J Neurooncol. 1984;2(2):147–51.
Young H, Kaplan A, Regelson W. Immunotherapy with autologous white cell infusions (“lymphocytes”) in the treatment of recurrrent glioblastoma multiforme: a preliminary report. Cancer. 1977;40(3):1037–44.
Brown CE, Starr R, Aguilar B, Shami AF, Martinez C, D’Apuzzo M, Barish ME, Forman SJ, Jensen MC. Stem-like tumor-initiating cells isolated from IL13Ralpha2 expressing gliomas are targeted and killed by IL13-zetakine-redirected T cells. Clin Cancer Res. 2012;18(8):2199–209.
Morgan RA, Johnson LA, Davis JL, Zheng Z, Woolard KD, Reap EA, Feldman SA, Chinnasamy N, Kuan CT, Song H, et al. Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma. Hum Gene Ther. 2012;23(10):1043–53.
Ahmed N, Salsman VS, Kew Y, Shaffer D, Powell S, Zhang YJ, Grossman RG, Heslop HE, Gottschalk S. HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clin Cancer Res. 2010;16(2):474–85.
Miao H, Gale NW, Guo H, Qian J, Petty A, Kaspar J, Murphy AJ, Valenzuela DM, Yancopoulos G, Hambardzumyan D, et al. EphA2 promotes infiltrative invasion of glioma stem cells in vivo through cross-talk with Akt and regulates stem cell properties. Oncogene. 2015;34(5):558–67.
Mount CW, Majzner RG, Sundaresh S, Arnold EP, Kadapakkam M, Haile S, Labanieh L, Hulleman E, Woo PJ, Rietberg SP, et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M(+) diffuse midline gliomas. Nat Med. 2018;24(5):572–9.
Golinelli G, Grisendi G, Prapa M, Bestagno M, Spano C, Rossignoli F, Bambi F, Sardi I, Cellini M, Horwitz EM, et al. Targeting GD2-positive glioblastoma by chimeric antigen receptor empowered mesenchymal progenitors. Cancer Gene Ther. 2018;27:558–70.
Majzner RG, Theruvath JL, Nellan A, Heitzeneder S, Cui Y, Mount CW, Rietberg SP, Linde MH, Xu P, Rota C, et al. CAR T cells targeting B7-H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin Cancer Res. 2019;25(8):2560–74.
Wang D, Starr R, Chang WC, Aguilar B, Alizadeh D, Wright SL, Yang X, Brito A, Sarkissian A, Ostberg JR, et al. Chlorotoxin-directed CAR T cells for specific and effective targeting of glioblastoma. Sci Transl Med. 2020. https://doi.org/10.1126/scitranslmed.aaw2672.
Kuwana Y, Asakura Y, Utsunomiya N, Nakanishi M, Arata Y, Itoh S, Nagase F, Kurosawa Y. Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions. Biochem Biophys Res Commun. 1987;149(3):960–8.
Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci USA. 1989;86(24):10024–8.
Abate-Daga D, Davila ML. CAR models: next-generation CAR modifications for enhanced T-cell function. Mol Ther Oncolytics. 2016;3:16014.
Chmielewski M, Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther. 2015;15(8):1145–54.
Garrido F, Aptsiauri N, Doorduijn EM, Garcia Lora AM, van Hall T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr Opin Immunol. 2016;39:44–51.
Watanabe N, Bajgain P, Sukumaran S, Ansari S, Heslop HE, Rooney CM, Brenner MK, Leen AM, Vera JF. Fine-tuning the CAR spacer improves T-cell potency. Oncoimmunology. 2016;5(12):e1253656.
Morin SO, Giroux V, Favre C, Bechah Y, Auphan-Anezin N, Roncagalli R, Mege JL, Olive D, Malissen M, Nunes JA. In the absence of its cytosolic domain, the CD28 molecule still contributes to T cell activation. Cell Mol Life Sci. 2015;72(14):2739–48.
Guedan S, Posey AD Jr, Shaw C, Wing A, Da T, Patel PR, McGettigan SE, Casado-Medrano V, Kawalekar OU, Uribe-Herranz M, et al. Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation. JCI Insight. 2018;3(1):e96976.
Tamada K, Geng D, Sakoda Y, Bansal N, Srivastava R, Li Z, Davila E. Redirecting gene-modified T cells toward various cancer types using tagged antibodies. Clin Cancer Res. 2012;18(23):6436–45.
Cho JH, Collins JJ, Wong WW. Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell. 2018;173(6):1421.e4116-1438.e1411.
Han X, Wang Y, Wei J, Han W. Multi-antigen-targeted chimeric antigen receptor T cells for cancer therapy. J Hematol Oncol. 2019;12(1):128.
Hegde M, Corder A, Chow KK, Mukherjee M, Ashoori A, Kew Y, Zhang YJ, Baskin DS, Merchant FA, Brawley VS, et al. Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Mol Ther. 2013;21(11):2087–101.
Bielamowicz K, Fousek K, Byrd TT, Samaha H, Mukherjee M, Aware N, Wu MF, Orange JS, Sumazin P, Man TK, et al. Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro-oncology. 2018;20(4):506–18.
Hegde M, Mukherjee M, Grada Z, Pignata A, Landi D, Navai SA, Wakefield A, Fousek K, Bielamowicz K, Chow KK, et al. Tandem CAR T cells targeting HER2 and IL13Ralpha2 mitigate tumor antigen escape. J Clin Invest. 2016;126(8):3036–52.
Lanitis E, Poussin M, Klattenhoff AW, Song D, Sandaltzopoulos R, June CH, Powell DJ Jr. Chimeric antigen receptor T Cells with dissociated signaling domains exhibit focused antitumor activity with reduced potential for toxicity in vivo. Cancer Immunol Res. 2013;1(1):43–53.
Roybal KT, Rupp LJ, Morsut L, Walker WJ, McNally KA, Park JS, Lim WA. Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell. 2016;164(4):770–9.
Morsut L, Roybal KT, **ong X, Gordley RM, Coyle SM, Thomson M, Lim WA. Engineering customized cell sensing and response behaviors using synthetic notch receptors. Cell. 2016;164(4):780–91.
Roybal KT, Williams JZ, Morsut L, Rupp LJ, Kolinko I, Choe JH, Walker WJ, McNally KA, Lim WA. Engineering T cells with customized therapeutic response programs using synthetic notch receptors. Cell. 2016;167(2):419.e416-432.e416.
Krenciute G, Prinzing BL, Yi Z, Wu MF, Liu H, Dotti G, Balyasnikova IV, Gottschalk S. Transgenic expression of IL15 improves antiglioma activity of IL13Ralpha2-CAR T cells but results in antigen loss variants. Cancer Immunol Res. 2017;5(7):571–81.
Shum T, Omer B, Tashiro H, Kruse RL, Wagner DL, Parikh K, Yi Z, Sauer T, Liu D, Parihar R, et al. Constitutive signaling from an engineered IL7 receptor promotes durable tumor elimination by tumor-redirected T cells. Cancer Discov. 2017;7(11):1238–47.
Zimmermann K, Kuehle J, Dragon AC, Galla M, Kloth C, Rudek LS, Sandalcioglu IE, Neyazi B, Moritz T, Meyer J, et al. Design and characterization of an “all-in-one” lentiviral vector system combining constitutive anti-GD2 CAR expression and inducible cytokines. Cancers. 2020;12(2):375.
O’Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, Martinez-Lage M, Brem S, Maloney E, Shen A, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9(399):eaaa0984.
Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014;20(2):119–22.
Frey N, Porter D. Cytokine release syndrome with chimeric antigen receptor T cell therapy. Biol Blood Marrow Transpl. 2019;25(4):e123–7.
Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–18.
Stavrou M, Philip B, Traynor-White C, Davis CG, Onuoha S, Cordoba S, Thomas S, Pule M. A rapamycin-activated caspase 9-based suicide gene. Mol Ther. 2018;26(5):1266–76.
Yi Z, Prinzing BL, Cao F, Gottschalk S, Krenciute G. Optimizing EphA2-CAR T cells for the adoptive immunotherapy of glioma. Mol Ther Methods Clin Dev. 2018;9:70–80.
Paszkiewicz PJ, Frassle SP, Srivastava S, Sommermeyer D, Hudecek M, Drexler I, Sadelain M, Liu L, Jensen MC, Riddell SR, et al. Targeted antibody-mediated depletion of murine CD19 CAR T cells permanently reverses B cell aplasia. J Clin Invest. 2016;126(11):4262–72.
Kao RL, Truscott LC, Chiou TT, Tsai W, Wu AM, De Oliveira SN. A cetuximab-mediated suicide system in chimeric antigen receptor-modified hematopoietic stem cells for cancer therapy. Hum Gene Ther. 2019;30(4):413–28.
Casucci M, Nicolis di Robilant B, Falcone L, Camisa B, Norelli M, Genovese P, Gentner B, Gullotta F, Ponzoni M, Bernardi M, et al. CD44v6-targeted T cells mediate potent antitumor effects against acute myeloid leukemia and multiple myeloma. Blood. 2013;122(20):3461–72.
Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, Ostberg JR, Blanchard MS, Kilpatrick J, Simpson J, et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375(26):2561–9.
Sattiraju A, Solingapuram Sai KK, Xuan A, Pandya DN, Almaguel FG, Wadas TJ, Herpai DM, Debinski W, Mintz A. IL13RA2 targeted alpha particle therapy against glioblastomas. Oncotarget. 2017;8(26):42997–3007.
Thaci B, Brown CE, Binello E, Werbaneth K, Sampath P, Sengupta S. Significance of interleukin-13 receptor alpha 2-targeted glioblastoma therapy. Neuro-oncology. 2014;16(10):1304–12.
Brown CE, Badie B, Barish ME, Weng L, Ostberg JR, Chang WC, Naranjo A, Starr R, Wagner J, Wright C, et al. Bioactivity and safety of IL13Ralpha2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin Cancer Res. 2015;21(18):4062–72.
Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–8.
Chistiakov DA, Chekhonin IV, Chekhonin VP. The EGFR variant III mutant as a target for immunotherapy of glioblastoma multiforme. Eur J Pharmacol. 2017;810:70–82.
Goff SL, Morgan RA, Yang JC, Sherry RM, Robbins PF, Restifo NP, Feldman SA, Lu YC, Lu L, Zheng Z, et al. Pilot trial of adoptive transfer of chimeric antigen receptor-transduced T cells targeting EGFRvIII in patients with glioblastoma. J Immunother. 2019;42(4):126–35.
Sampson JH, Choi BD, Sanchez-Perez L, Suryadevara CM, Snyder DJ, Flores CT, Schmittling RJ, Nair SK, Reap EA, Norberg PK, et al. EGFRvIII mCAR-modified T-cell therapy cures mice with established intracerebral glioma and generates host immunity against tumor-antigen loss. Clin Cancer Res. 2014;20(4):972–84.
Schonfeld K, Sahm C, Zhang C, Naundorf S, Brendel C, Odendahl M, Nowakowska P, Bonig H, Kohl U, Kloess S, et al. Selective inhibition of tumor growth by clonal NK cells expressing an ErbB2/HER2-specific chimeric antigen receptor. Mol Ther. 2015;23(2):330–8.
Ahmed N, Brawley V, Hegde M, Bielamowicz K, Kalra M, Landi D, Robertson C, Gray TL, Diouf O, Wakefield A, et al. HER2-specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol. 2017;3(8):1094–101.
Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4):843–51.
Liu X, Zhang N, Shi H. Driving better and safer HER2-specific CARs for cancer therapy. Oncotarget. 2017;8(37):62730–41.
Liu X, Jiang S, Fang C, Yang S, Olalere D, Pequignot EC, Cogdill AP, Li N, Ramones M, Granda B, et al. Affinity-tuned ErbB2 or EGFR chimeric antigen receptor T cells exhibit an increased therapeutic index against tumors in mice. Cancer Res. 2015;75(17):3596–607.
Ahmed N, Brawley VS, Hegde M, Robertson C, Ghazi A, Gerken C, Liu E, Dakhova O, Ashoori A, Corder A, et al. Human epidermal growth factor receptor 2 (HER2)—specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol. 2015;33(15):1688–96.
Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, Huls MH, Liu E, Gee AP, Mei Z, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14(11):1264–70.
Wang LF, Fokas E, Bieker M, Rose F, Rexin P, Zhu Y, Pagenstecher A, Engenhart-Cabillic R, An HX. Increased expression of EphA2 correlates with adverse outcome in primary and recurrent glioblastoma multiforme patients. Oncol Rep. 2008;19(1):151–6.
Wykosky J, Gibo DM, Stanton C, Debinski W. EphA2 as a novel molecular marker and target in glioblastoma multiforme. Mol Cancer Res. 2005;3(10):541–51.
Chow KK, Naik S, Kakarla S, Brawley VS, Shaffer DR, Yi Z, Rainusso N, Wu MF, Liu H, Kew Y, et al. T cells redirected to EphA2 for the immunotherapy of glioblastoma. Mol Ther. 2013;21(3):629–37.
Sorokin M, Kholodenko I, Kalinovsky D, Shamanskaya T, Doronin I, Konovalov D, Mironov A, Kuzmin D, Nikitin D, Deyev S, et al. RNA sequencing-based identification of ganglioside GD2-positive cancer phenotype. Biomedicines. 2020;8(6):142.
Zhou Z, Luther N, Ibrahim GM, Hawkins C, Vibhakar R, Handler MH, Souweidane MM. B7-H3, a potential therapeutic target, is expressed in diffuse intrinsic pontine glioma. J Neurooncol. 2013;111(3):257–64.
Souweidane MM, Kramer K, Pandit-Taskar N, Zhou Z, Haque S, Zanzonico P, Carrasquillo JA, Lyashchenko SK, Thakur SB, Donzelli M, et al. Convection-enhanced delivery for diffuse intrinsic pontine glioma: a single-centre, dose-escalation, phase 1 trial. Lancet Oncol. 2018;19(8):1040–50.
Kramer K, Kushner BH, Modak S, Pandit-Taskar N, Smith-Jones P, Zanzonico P, Humm JL, Xu H, Wolden SL, Souweidane MM, et al. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J Neurooncol. 2010;97(3):409–18.
Nguyen P, Okeke E, Clay M, Haydar D, Justice J, O’Reilly C, Pruett-Miller S, Papizan J, Moore J, Zhou S, et al. Route of 41BB/41BBL costimulation determines effector function of B7-H3-CAR.CD28ζ T cells. Mol Ther Oncolytics. 2020;18:202–14.
Dardevet L, Rani D, Aziz TA, Bazin I, Sabatier JM, Fadl M, Brambilla E, De Waard M. Chlorotoxin: a helpful natural scorpion peptide to diagnose glioma and fight tumor invasion. Toxins. 2015;7(4):1079–101.
** L, Ge H, Long Y, Yang C, Chang YE, Mu L, Sayour EJ, De Leon G, Wang QJ, Yang JC, et al. CD70, a novel target of CAR T-cell therapy for gliomas. Neuro-oncology. 2018;20(1):55–65.
Ge H, Mu L, ** L, Yang C, Chang YE, Long Y, DeLeon G, Deleyrolle L, Mitchell DA, Kubilis PS, et al. Tumor associated CD70 expression is involved in promoting tumor migration and macrophage infiltration in GBM. Int J Cancer. 2017;141(7):1434–44.
Wang QJ, Yu Z, Hanada KI, Patel K, Kleiner D, Restifo NP, Yang JC. Preclinical evaluation of chimeric antigen receptors targeting CD70-expressing cancers. Clin Cancer Res. 2017;23(9):2267–76.
Vora P, Venugopal C, Salim SK, Tatari N, Bakhshinyan D, Singh M, Seyfrid M, Upreti D, Rentas S, Wong N, et al. The rational development of CD133-targeting immunotherapies for glioblastoma. Cell Stem Cell. 2020;26(6):832.e836-844.e836.
Wang Y, Chen M, Wu Z, Tong C, Dai H, Guo Y, Liu Y, Huang J, Lv H, Luo C, et al. CD133-directed CAR T cells for advanced metastasis malignancies: a phase I trial. Oncoimmunology. 2018;7(7):e1440169.
**e Q, Bradley R, Kang L, Koeman J, Ascierto ML, Worschech A, De Giorgi V, Wang E, Kefene L, Su Y, et al. Hepatocyte growth factor (HGF) autocrine activation predicts sensitivity to MET inhibition in glioblastoma. Proc Natl Acad Sci USA. 2012;109(2):570–5.
Petterson SA, Dahlrot RH, Hermansen SK, Munthe SK, Gundesen MT, Wohlleben H, Rasmussen T, Beier CP, Hansen S, Kristensen BW. High levels of c-Met is associated with poor prognosis in glioblastoma. J Neurooncol. 2015;122(3):517–27.
Qin Y, Musket A, Kou J, Preiszner J, Tschida B, Qin A, Land C, Staal B, Kang L, Tanner K, et al. Overexpression of HGF/MET axis along with p53 inhibition induces de novo glioma formation in mice. Neuro Oncol Adv. 2020. https://doi.org/10.1093/noajnl/vdaa067.
Johnson J, Ascierto ML, Mittal S, Newsome D, Kang L, Briggs M, Tanner K, Marincola FM, Berens ME, Vande Woude GF, et al. Genomic profiling of a hepatocyte growth factor-dependent signature for MET-targeted therapy in glioblastoma. J Transl Med. 2015;13:306.
Kou J, Musich PR, Staal B, Kang L, Qin Y, Yao ZQ, Zhang B, Wu W, Tam A, Huang A, et al. Differential responses of MET activations to MET kinase inhibitor and neutralizing antibody. J Transl Med. 2018;16(1):253.
Cloughesy T, Finocchiaro G, Belda-Iniesta C, Recht L, Brandes AA, Pineda E, Mikkelsen T, Chinot OL, Balana C, Macdonald DR, et al. Randomized, double-blind, placebo-controlled, multicenter phase II study of onartuzumab plus bevacizumab versus placebo plus bevacizumab in patients with recurrent glioblastoma: efficacy, safety, and hepatocyte growth factor and O(6)-methylguanine-DNA methyltransferase biomarker analyses. J Clin Oncol. 2017;35(3):343–51.
Akhavan D, Alizadeh D, Wang D, Weist MR, Shepphird JK, Brown CE. CAR T cells for brain tumors: lessons learned and road ahead. Immunol Rev. 2019;290(1):60–84.
Gajewski TF, Meng Y, Blank C, Brown I, Kacha A, Kline J, Harlin H. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–45.
Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39.
Chong EA, Melenhorst JJ, Lacey SF, Ambrose DE, Gonzalez V, Levine BL, June CH, Schuster SJ. PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR. Blood. 2017;129(8):1039–41.
Khasraw M, Reardon DA, Weller M, Sampson JH. PD-1 inhibitors: do they have a future in the treatment of glioblastoma? Clin Cancer Res. 2020. https://doi.org/10.1158/1078-0432.CCR-20-1135.
Filley AC, Henriquez M, Dey M. Recurrent glioma clinical trial, CheckMate-143: the game is not over yet. Oncotarget. 2017;8(53):91779–94.
Maxwell R, Jackson CM, Lim M. Clinical trials investigating immune checkpoint blockade in glioblastoma. Curr Treat Options Oncol. 2017;18(8):51.
Cloughesy TF, Mochizuki AY, Orpilla JR, Hugo W, Lee AH, Davidson TB, Wang AC, Ellingson BM, Rytlewski JA, Sanders CM, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477–86.
Mathios D, Kim JE, Mangraviti A, Phallen J, Park CK, Jackson CM, Garzon-Muvdi T, Kim E, Theodros D, Polanczyk M, et al. Anti-PD-1 antitumor immunity is enhanced by local and abrogated by systemic chemotherapy in GBM. Sci Transl Med. 2016;8(370):370ra180.
Wang E, Cesano A, Butterfield LH, Marincola F. Improving the therapeutic index in adoptive cell therapy: key factors that impact efficacy. J Immunother Cancer. 2020;8(2):e001619.
Ren J, Liu X, Fang C, Jiang S, June CH, Zhao Y. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin Cancer Res. 2017;23(9):2255–66.
Ren J, Zhao Y. Advancing chimeric antigen receptor T cell therapy with CRISPR/Cas9. Protein Cell. 2017;8(9):634–43.
Liu X, Zhang Y, Cheng C, Cheng AW, Zhang X, Li N, **a C, Wei X, Liu X, Wang H. CRISPR-Cas9-mediated multiplex gene editing in CAR-T cells. Cell Res. 2017;27(1):154–7.
Li L, Gao Y, Srivastava R, Wang W, **ong Q, Fang Z, Pelayo A, Denson C, Goswami A, Harari-Steinfeld R, et al. Lentiviral delivery of combinatorial CAR/CRISPRi circuit into human primary T cells is enhanced by TBK1/IKKvarepsilon complex inhibitor BX795. J Transl Med. 2020;18(1):363.
Stadtmauer EA, Fraietta JA, Davis MM, Cohen AD, Weber KL, Lancaster E, Mangan PA, Kulikovskaya I, Gupta M, Chen F, et al. CRISPR-engineered T cells in patients with refractory cancer. Science. 2020. https://doi.org/10.1126/science.aba7365.
Wei J, Long L, Zheng W, Dhungana Y, Lim SA, Guy C, Wang Y, Wang Y-D, Qian C, Xu B, et al. Targeting REGNASE-1 programs long-lived effector T cells for cancer therapy. Nature. 2019;576(7787):471–6.
Acknowledgements
Figures were created in BioRender.com.
Funding
This work was supported by the ETSU start-up fund, a Department of Defense Ideal Award CA180296 (to QX), and the Assisi Foundation of Memphis, and the American Lebanese Syrian Associated Charities (ALSAC) (to GK). QX, GK have patent applications in the field of immunotherapy.
Author information
Authors and Affiliations
Contributions
QX and GK developed the concept; CAL, PRM, DH, GK and QX wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Land, C.A., Musich, P.R., Haydar, D. et al. Chimeric antigen receptor T-cell therapy in glioblastoma: charging the T cells to fight. J Transl Med 18, 428 (2020). https://doi.org/10.1186/s12967-020-02598-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-020-02598-0