Abstract
Autophagy is an evolutionarily conserved process that plays a role in regulating homeostasis under physiological conditions. However, dysregulation of autophagy is observed in the development of human diseases, especially cancer. Autophagy has reciprocal functions in cancer and may be responsible for either survival or death. Hepatocellular carcinoma (HCC) is one of the most lethal and common malignancies of the liver, and smoking, infection, and alcohol consumption can lead to its development. Genetic mutations and alterations in molecular processes can exacerbate the progression of HCC. The function of autophagy in HCC is controversial and may be both tumor suppressive and tumor promoting. Activation of autophagy may affect apoptosis in HCC and is a regulator of proliferation and glucose metabolism. Induction of autophagy may promote tumor metastasis via induction of EMT. In addition, autophagy is a regulator of stem cell formation in HCC, and pro-survival autophagy leads to cancer cell resistance to chemotherapy and radiotherapy. Targeting autophagy impairs growth and metastasis in HCC and improves tumor cell response to therapy. Of note, a large number of signaling pathways such as STAT3, Wnt, miRNAs, lncRNAs, and circRNAs regulate autophagy in HCC. Moreover, regulation of autophagy (induction or inhibition) by antitumor agents could be suggested for effective treatment of HCC. In this paper, we comprehensively review the role and mechanisms of autophagy in HCC and discuss the potential benefit of targeting this process in the treatment of the cancer.
Graphical abstract

Video Abstract
Similar content being viewed by others
Introduction
Autophagy is an evolutionarily conserved mechanism with a potential role in organelle and protein turnover that may also be involved in regulating metabolism and controlling cell quality [1, 2]. The process of autophagy is dependent on lysosomes and the main goal of autophagy is to provide nutrients and energy, which can be achieved by the degradation of cytoplasmic substituents. In addition, autophagy is crucial in the process of eliminating toxic proteins and defective organelles during the aging process [3]. There is increasing evidence that autophagy can influence cellular specialization, differentiation, protein trafficking, and unconventional secretion [4,5,6]. Autophagy is classified into three categories: Macroautophagy, microautophagy, and chaperone-mediated autophagy [7]. In this review, we focus on macroautophagy and refer to it simply as autophagy. The basic level of autophagy is required under normal conditions, but its induction can be mediated by metabolic changes [8, 56]. The incidence rate of HCC is two to four times higher in men compared to women [56]. HCC is considered the sixth most common cancer worldwide and up to 500,000 new cases are diagnosed annually, which are responsible for a high mortality rate, making it the third leading cause of death among tumors [60, 61]. The incidence rate of HCC is estimated to be 3.6–10.5 per 100,000, which may increase to 16 per 100,000 worldwide [62, 63]. The survival rate of HCC patients is low, with 5% of them surviving more than 5 years after diagnosis. This is related to the late diagnosis of HCC patients and only 15% of patients are eligible for liver transplantation and surgery. 50% of them undergo non-surgical therapies and 35% or more receive the best treatment during diagnosis [63]. Risk factors for HCC vary and may include alcohol consumption, hepatitis virus infection, cirrhosis, and nonalcoholic fatty liver disease [64].
The progression of HCC depends on the interaction of some mechanisms and pathways at the molecular level. Phosphorylation of Fis1 occurs through Met to mediate mitochondrial fission and promote HCC migration [65]. CD44 can increase the expression of YAP to promote HCC progression [66]. It seems that obesity is a driving force for HCC progression. PI3Kγ ablation decreases HCC proliferation and reduces insulinemia, steatosis, and inflammatory cytokine concentration [67]. ZRANB1, as a deubiquitinate, is involved in the increasing progression and malignancy of HCC and, to this end, increases LOXL2 expression to promote tumor metastasis [120]. CCAT2 mediates unfavorable prognosis in HCC [121] and may increase the expression of NDRG1 and MDM2 in promoting tumor progression [122, 123]. Overexpression of CCAT2 is observed in HCC samples and leads to advanced stage and venous invasion. CCAT2 decreases the expression of miR-4496 in the cytoplasm to promote ATG expression, trigger autophagy, and enhance metastasis of HCC cells [124]. One of the best-known regulators of autophagy is Beclin-1, which was mentioned in the introduction. BMP4 stimulates c-Jun N-terminal kinase (JNK) signaling to increase the expression of Beclin-1 in autophagy induction, leading to a marked increase in HCC cell metastasis and invasion [125].
Glycochenodeoxycholate (GCDC) is one of the components of bile acid and can cause liver damage due to its hydrophobic acidic nature [80, 126,127,128,129,130]. Hydrophobic bile acid can lead to apoptosis and necrosis in hepatocytes [131]. In HCC, GCDC has been associated with an increase in progression and metastasis of HCC cells. GCDC promotes AMPK expression to inhibit mTOR signaling, leading to induction of autophagy and increased tumor cell invasion [132]. It appears that a lack of autophagy, which may be mediated by caveolin-1, leads to induction of angiogenesis and acceleration of metastasis in HCC [133].
Autophagy and EMT
EMT is the closest mechanism associated with tumor cell invasion and metastasis, as well as their resistance to therapy, and is characterized by morphological and physiological changes such as loss of cell polarity, disruption of intracellular junctions, increase in growth and invasion, increase in N-cadherin and vimentin levels, and decrease in E-cadherin [134,135,136,137]. Fluid shear stress (FSS) increases autophagosome formation and promotes the expression of Beclin-1, ATG7, and LC3II to mediate EMT and enhance HCC cell metastasis [138].
Autophagy and anoikis
Anoikis is a type of detachment from the extracellular matrix (ECM) [139] and the development of resistance to anoikis is critical for cancer cells to enhance their invasion and metastasis [140]. During anoikis resistance, there may be activation of autophagy, which is responsible for adaptation to stressful conditions such as oxidative stress, starvation, hypoxia, and metabolic reprogramming [141]. There is growing evidence of a link between autophagy and anoikis resistance in HCC. Acidic extracellular pH inhibits mTOR signaling via upregulation of AMP-protein kinase (AMPK) expression to induce autophagy. Moreover, miR-3663-3p is downregulated at acidic extracellular pH to induce autophagy, which promotes anoikis resistance in HCC [142]. Therefore, autophagy stimulates anoikis resistance in increasing progression and metastasis of HCC cells. miR-30a reduces the levels of Beclin-1 and ATG5 to suppress autophagy and anoikis resistance and limit metastasis of HCC cells [143]. AEG-1 stimulates phosphorylation of ULK1 to stimulate autophagy in mediating anoikis resistance and enhancing HCC cell invasion [144]. Based on these studies, targeting autophagy and related molecular signaling pathways is of importance in treating HCC and reducing cancer cell metastasis (Fig. 4) [145].
Targeting autophagy to regulate metastasis in HCC. Anoikis resistance and EMT as the two most important factors in regulating HCC cell invasion are influenced by autophagy mechanism. When autophagy has an oncogenic function, its induction by Beclin-1 and AMPK/mTOR signaling pathway may lead to EMT induction, which promotes tumor metastasis
Regulation of proliferation by autophagy has shown that interfering with this mechanism may provide new insights into how tumor survival is affected. Most importantly, autophagy has been found to be related to metastasis as another feature of HCC. Autophagy may act as a trigger/inhibitor of metastasis in HCC. Overexpression of ATG2A and ATG14 leads to autophagy and a subsequent increase in metastasis of HCC cells, whereas inhibition of the Beclin-1/autophagy axis by SOCS5 promotes metastasis. Since metastasis is closely related to the malignancy of tumor cells and may also mediate chemoresistance, induction of autophagy in HCC cells, when acting as a tumor suppressor, may contribute significantly to the impairment of carcinogenesis.
Targeting autophagy for regulating drug resistance
Basic evolution of drug resistance in HCC
The process of chemoresistance in HCC has been challenging in recent years, and prevention of mitochondrial respiration and stimulation of oxidative stress have been responsible for overcoming chemoresistance [146]. Overexpression of ICMT prevents apoptosis and induces doxorubicin resistance in HCC [147]. Overexpression of Nrf2 induces chemoresistance in HCC and metformin suppresses Nrf2-mediated glycolysis, thereby increasing the drug sensitivity of cancer cells [148]. Downregulation of USP7 reduces HCC cell growth and metastasis and is responsible for drug sensitivity [149]. Glyochenodeoxycholic acid stimulates STAT3 signaling to promote stem cell formation of HCC cells and mediates drug resistance [150]. Therefore, molecular pathways contribute to HCC cell progression and mediate drug resistance, so the role of autophagy mechanism in this aspect is under investigation.
Related molecular pathways
Upregulation of CD24 is observed in HCC cells and those resistant to sorafenib chemotherapy. CD24 increases the expression of PP2A and suppresses the mTOR/Akt axis to induce autophagy, which triggers sorafenib resistance in HCC [151]. The redox status of tumor cells is different from that of normal cells [152], and overexpression of antioxidant factors can enhance the progression of HCC cells [153]. Ferroptosis is a type of programmed oxidative cell death characterized by stimulation of the Fenton reaction, which promotes the formation of ROS and increases the accumulation of lipid peroxidation products [154]. Sorafenib can cause depletion of GSH, triggering ferroptosis in HCC [155, 156]. Overexpression of CISD2 in HCC is responsible for sorafenib resistance in tumor cells. Silencing of CISD2 stimulates apoptosis and uncontrolled autophagy in HCC and increases sorafenib-mediated ferroptosis in tumor cells [157]. Inhibition of pro-survival autophagy is important for increasing sorafenib sensitivity of HCC cells. Downregulation of miR-541 in HCC leads to sorafenib resistance and suppresses proliferation, metastasis, and autophagy in vitro and in vivo. miR-541 decreases ATG2A and RAB1B levels to inhibit autophagy and increase sorafenib sensitivity of HCC cells [158]. Although the function of miR-541 is critical for enhancing drug sensitivity in HCC, upregulation of miR-25 leads to sorafenib resistance. miR-25 decreases FBXW7 expression to trigger autophagy in mediating sorafenib resistance in HCC [159].
The expression of Rage is found in various types of cells [160] and has been found to be associated with the progression of inflammatory diseases such as cancer [160,161,162]. Recently, the role of Rage in regulating the progression of HCC cells has been investigated. CircRNA-101368 increases Rage levels and thus promotes HCC progression [163]. The upregulation of Rage in HCC promotes the growth rate of HCC cells and stimulates sorafenib resistance. Loss of Rage expression leads to stimulation of AMPK signaling to reduce mTOR expression in autophagy induction and promote sorafenib response in HCC [164]. Yes-associated protein (YAP) is one of the new targets in HCC therapy and KAT6A increases the expression of YAP, which promotes HCC progression and mediates sorafenib resistance [165]. Cinacalcet inhibits the YAP /TAZ axis to prevent HCC progression [166]. Moreover, overexpression of YAP leads to EMT induction, which enhances HCC metastasis [167]. An experiment has shown that overexpression of YAP can lead to drug resistance in HCC. Inhibition of YAP increases drug sensitivity in HCC via mediating autophagy-induced cell death. Knockdown of YAP leads to increased RAC1-mediated ROS generation to suppress mTOR signaling, resulting in autophagy induction and chemosensitivity in HCC [168].
Autophagy and apoptosis in chemoresistance
The major pathway by which chemotherapeutic agents exert cytotoxicity on tumor cells is the induction of apoptosis, which can be stimulated by both mitochondria and endoplasmic reticulum stress (ER). ER Stress leads to activation of the UPR, which can mediate apoptosis [169, 170]. Moreover, ER stress can induce both apoptosis and autophagy, and compounds targeting ER stress are of interest for the treatment of disease and cancer [171]. In HCC cells exposed to sorafenib, overexpression of IRE1 is critical for the induction of autophagy via the ER stress signaling pathway. Moreover, activation of autophagy reduces ER stress-induced apoptosis in HCC. Therefore, inhibition of autophagy and its targeting may improve the potential of chemotherapeutic agents in apoptosis induction in HCC [172,173,174]. Table 2 summarizes the role of autophagy in drug resistance in HCC.
The process of chemoresistance is highly complicated and it is impossible to target all the molecular pathways involved in this condition, but it is possible to adopt some of the most important mechanisms in this case. Because of the interaction of autophagy with other cell death mechanisms, its induction may mediate ferroptosis in enhancing drug sensitivity, while inhibition of autophagy by miR-541 is critical in preventing chemoresistance, again demonstrating the dual function of autophagy in this case. One of the drawbacks of the current studies is that they did not focus on other chemotherapeutic agents such as doxorubicin, paclitaxel, and others, but focused on sorafenib. Future studies should therefore pay particular attention to the role of autophagy in the resistance of HCC to the above agents.
Targeting autophagy for regulating radioresistance
Modulation of radioresistance in HCC is of importance because it is a conventional therapy for HCC. If the potential of therapy is reduced due to resistance, a functional analysis of genes should be performed to reveal the role of factors involved in progression and resistance. Radiotherapy stimulates CD8+ T cell function to impair HCC progression, and sorafenib is considered an inhibitor of radioresistance [175]. HCC cells overexpressing γ GCSh prevent apoptosis, which is beneficial for inducing radioresistance [176]. This section focuses on the role of autophagy in regulating radioresistance in HCC. NEAT1 is a regulator of autophagy in cancer and its downregulation can stimulate autophagy and ferroptosis [177]. The upregulation of NEAT1 in HCC has been associated with an important property known as radioresistance. This effect is mediated by overexpression of gamma-aminobutyric acid receptor-associated protein (GABARAP) and induction of autophagy [178]. The effect of autophagy on radioresistance in HCC is uncertain. Irradiation of HCC cells leads to autophagy induction, and oxaliplatin promotes autophagy activation by increasing the cytotoxicity of radiotherapy in the treatment of HCC [179]. However, most studies have focused on the role of autophagy as a pro-survival mechanism in triggering radioresistance. ASPP is an apoptosis regulatory protein and has three members, including ASPP1, ASPP2, and iASPP. ASPP2 is downregulated in HCC due to its methylation [180, 181]. Moreover, ASPP2 prevents autophagy to increase cell response to RAS [182]. Downregulation of ASPP2 may lead to an increase in HCC cell survival. Low expression of ASPP2 leads to overexpression of Beclin-1 and induction of autophagy [183]. Previous experiments have investigated the role of ASPP2 in the regulation of autophagy and its association with drug resistance, and future studies may be warranted to investigate its role in radioresistance. RAD001 is an inhibitor of mTOR signaling that stimulates autophagy as a pro-death mechanism to enhance autophagy and the efficacy of radiotherapy in combating HCC [184]. Saikosaponin-d promotes LC3 levels and increases autophagosome formation to stimulate autophagy and increase the radiosensitivity of HCC cells [185]. The biological effects of irradiation are mediated by the formation of ROS [186]. Irradiation can increase the formation of ROS and mediates oxidative stress [187]. Thus, when the levels of ROS decrease, the sensitivity of HCC cells to radiotherapy decreases. URI1 induces AMPK phosphorylation to increase forkhead box O3 (FOXO3) levels to trigger autophagy via increased autophagosome formation and prevent ROS formation by radiotherapy in HCC [188]. According to these studies, targeting autophagy and regulating related molecular signaling pathways are important for modulating the response of HCC cells to radiotherapy (Fig. 5) [189,190,191,192,193].
Autophagy, drug resistance, and radioresistance in HCC. Based on the function of autophagy in regulating proliferation and metastasis of HCC cells, this metabolic pathway may be involved in regulating radio- and chemoresistance of HCC cells. The resistance of HCC cells to sorafenib and oxaliplatin is tightly regulated by the autophagy mechanism. Moreover, activation of autophagy by NEAT1 may lead to radioresistance. More importantly, autophagy can reduce the levels of ROS in triggering radioresistance
Although the focus of many studies has been on drug resistance, there have also been attempts to demonstrate the role of autophagy in mediating radioresistance in HCC. Most studies show that induction of cytotoxic autophagy is important in mediating radiosensitivity. One of the most important aspects is the use of chemotherapeutic agents such as oxaliplatin together with radiotherapy to stimulate pro-death autophagy and reduce HCC progression.
Anticancer agents modulating autophagy in hepatocellular carcinoma
Synthetic drugs
The use of anticancer agents, other than chemotherapeutic agents, is an increasing trend in the treatment of HCC as resistance to conventional therapies develops [194,195,196]. Shikonin is an inhibitor of HCC progression and suppresses the PI3K/Akt/mTOR axis to stimulate both apoptosis and autophagy to reduce cancer cell malignancy [197]. However, when an anti-cancer agent stimulates autophagy, it does not mean that autophagy promotes tumor progression. Thus, in HCC, myricetin stimulates both apoptosis and autophagy by affecting endoplasmic reticulum (ER) stress. Although activation of ER stress by myricetin stimulates apoptosis to reduce HCC progression, activation of autophagy is a supportive mechanism, and its inhibition may enhance the anticancer effect of myricetin anticancer therapy [198].
Imatinib is a tyrosine kinase inhibitor and impairs HCC cell metastasis by increasing NM23 expression (199). To improve the anti-cancer activity of imatinib against HCC, attempts have been made to combine it with other antitumor agents such as sulfasalazine and GNF-5 [200, 201]. Moreover, incorporation of imatinib into lactoferrin-modified PEGylated liquid crystalline nanostructures induces apoptosis in HCC via the mitochondrial pathway [202]. Imatinib prevents phosphorylation of protein kinase B (Akt) and promotes expression of p62 and suppresses progression of HCC in vitro and in vivo. Imatinib impairs HCC progression via regulating the above signaling pathways to inhibit autophagy [203].
Natural compounds
Another antitumor agent that is popular in HCC treatment is dioscin. Dioscin suppresses TGF-β1-induced EMT in HCC to reduce cancer cell metastasis [204]. In addition, dioscin increases Bax and caspase-3 levels and decreases Bcl-2 levels in apoptosis induction in HCC [205]. Dioscin stimulates apoptosis, autophagy, and DNA damage in HCC cells and reduces growth and metastasis. Dioscin increases the levels of Beclin-1 and LC-3 and decreases the levels of p-Akt and p-mTOR in inducing autophagy and promoting progression of HCC cells [206]. Another strategy is the simultaneous use of two anticancer drugs. For example, in one experiment, costunolide (CL) and dehydrocostuslactone (DCL) were used as two bioactive components of an extract of sesquiterpene lactones to treat HCC. CL and DCL promote the accumulation of LC3 and p62 to suppress autophagy and prevent HCC progression [207]. In the previous sections, it has been shown that inhibition of autophagy may be beneficial in improving apoptosis induction in HCC. However, when autophagy has a death-promoting function, its inhibition decreases apoptosis. Isoqerucetin stimulates the AMPK/mTOR/p70S6K axis to mediate apoptosis and autophagy in HCC. Inhibition of autophagy decreases apoptosis by lowering the Bax/Bcl-2 ratio and preventing capase-3 activation and PARP cleavage, confirming the anticancer effect of autophagy in HCC [208].
The most controversial part is that the function of autophagy in HCC is completely different depending on the conditions. For example, a previous experiment showed that imatinib suppresses autophagy in HCC therapy [203]. However, another experiment shows that ursodeoxycholic acid promotes LC3B expression to stimulate autophagy and thus prevent HCC progression [209]. Moreover, both studies have shown that autophagy is regulated in vitro and in vivo and has an effect on the progression of HCC.
Resveratrol is an effective anti-cancer agent against HCC and suppresses Akt signaling by increasing phosphatase and tensin homolog (PTEN) expression, thereby reducing the malignancy of HCC [210]. Resveratrol promotes anti-tumor immunity in HCC by reducing the number of CD8+ CD122+ Treg cells [211]. Moreover, resveratrol reduces Gli-1 expression in HCC suppression [212]. Resveratrol is able to upregulate the expression of p53 and suppress the PI3K/Akt signaling pathway to induce autophagy to prevent the progression of HCC [213]. Moreover, flavopereirine induces autophagy to prevent the progression of HCC [214]. Based on these studies, most of the anti-tumor agents targeting autophagy (induction or inhibition) in the treatment of HCC are natural products and have shown promising results [215,216,217]. However, natural products have poor bioavailability [218, 219], and their future application may be facilitated by using nanostructures for their delivery in the treatment of HCC and modulation of autophagy (Fig. 6; Table 3).
Regulation of autophagy by anti-cancer agents in the treatment of HCC. Molecular pathways related to autophagy, such as PI3K/Akt/mTOR, AMPK, and LC3II, are regulated by anticancer drugs. Of note, induction of autophagy by antitumor agents not only has a pro-death function, but sometimes also a pro-survival function, so in this case, inhibition of autophagy increases the potential of antitumor agents to induce apoptosis
This section has shown that modulation of autophagy by synthetic and natural agents offers a great opportunity for improved efficacy in the treatment of HCC. Sometimes, autophagy induced by anti-tumor agents has a pro-survival function, and in this case, inhibition of autophagy is suggested to enhance its efficacy in cancer therapy. One of the gaps in the current field is that studies have ignored the role of small molecules in regulating autophagy in HCC. Since the molecular signaling pathways related to autophagy such as ATGs, Beclin-1, and AMPK have been recognized and their structure has also been understood, it is highly recommended to develop new small molecules to affect autophagy in HCC therapy in the near future.
Conclusion and remarks
The autophagy mechanism is a molecular event in normal and cancer cells that has a completely different function depending on the context. In normal cells, the goal of autophagy is to break down aged organelles and decompose toxins. Therefore, a baseline level of autophagy or its induction may be beneficial for maintaining homeostasis under physiological conditions. However, as cancer cells progress, they may induce or inhibit autophagy depending on their condition to improve their survival rate. Since HCC is the most malignant and lethal liver cancer, the influence of autophagy on tumor cell progression needs to be emphasized. Autophagy has two distinct functions in HCC that impact survival and death. In the context of the pro-survival function, induction of autophagy can significantly enhance progression and viability of HCC cells, whereas pro-death autophagy impairs tumor progression. ER stress can mediate both apoptosis and autophagy in HCC cells, and inhibition of pro-survival autophagy has been shown to be beneficial in increasing ER stress-mediated apoptosis in tumor cells. AMPK, Beclin-1, and the Akt/mTOR pathway are the major regulators of autophagy that have been studied in HCC. Autophagy can increase HCC cell viability and prevent apoptosis, whereas death-promoting autophagy exerts a different function. Activation of autophagy may lead to increased invasion of HCC cells and induce EMT, whereas anti-cancer autophagy suppresses tumor cell migration. In addition, targeting autophagy is of interest to increase the sensitivity of HCC cells to drugs and radioactivity. Quercetin, resveratrol, and lycorine are among the compounds that target autophagy in the treatment of HCC. Induction of autophagy by anticancer agents does not indicate their tumor-suppressive effects, and sometimes inhibition of autophagy may improve the cytotoxicity of these agents in combating HCC. Future application of these findings in the clinic could greatly improve the prognosis, survival, and ability to treat HCC patients.
Basic research is important when it is translated into clinical practice in the treatment of patients. The current situation in the treatment of HCC patients is complicated because physicians face various problems in treating patients. Regardless of the lack of specific and highly sensitive tools for early diagnosis of HCC patients, their diagnosis in advanced and metastatic stages leads to difficulties in treatment, especially due to resistance to therapy. The clinical application of autophagy in these patients is that autophagy can be assessed prior to therapy to predict tumor cell response, and then a more effective therapeutic regimen can be applied to patients. Furthermore, since gene therapy has only recently entered the field of cancer therapy, it is possible that in the near future, factors related to autophagy can be targeted and modulated to enable more effective treatment of cancer patients.
Availability of data and materials
Not applicable.
References
Raudenska M, Balvan J, Masarik MJMC. Crosstalk between autophagy inhibitors and endosome-related secretory pathways: a challenge for autophagy-based treatment of solid cancers. Mol Cancer. 2021;20(1):1–27.
Kumariya S, Ubba V, Jha RK, Gayen JR. Autophagy in ovary and polycystic ovary syndrome: role, dispute and future perspective. Autophagy. 2021;17(10):2706–33.
Mizushima N. Physiological functions of autophagy. Curr Top Microbiol Immunol. 2009;335:71–84.
Clarke AJ, Simon AK. Autophagy in the renewal, differentiation and homeostasis of immune cells. Nat Rev Immunol. 2019;19(3):170–83.
Deretic V, Jiang S, Dupont N. Autophagy intersections with conventional and unconventional secretion in tissue development, remodeling and inflammation. Trends Cell Biol. 2012;22(8):397–406.
Salimi L, Akbari A, Jabbari N, Mojarad B, Vahhabi A, Szafert S, et al. Synergies in exosomes and autophagy pathways for cellular homeostasis and metastasis of tumor cells. Cell Biosci. 2020;10:64.
Wang Y, Mo Y, Peng M, Zhang S, Gong Z, Yan Q, et al. The influence of circular RNAs on autophagy and disease progression. Autophagy. 2022;18(2):240–53.
Kuma A, Mizushima N. Physiological role of autophagy as an intracellular recycling system: with an emphasis on nutrient metabolism. Semin Cell Dev Biol. 2010;21(7):683–90.
Fan C, Zhang S, Gong Z, Li X, **ang B, Deng H, et al. Emerging role of metabolic reprogramming in tumor immune evasion and immunotherapy. Sci China Life Sci. 2021;64(4):534–47.
Marino ML, Fais S, Djavaheri-Mergny M, Villa A, Meschini S, Lozupone F, et al. Proton pump inhibition induces autophagy as a survival mechanism following oxidative stress in human melanoma cells. Cell Death Dis. 2010;1(10):e87.
Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, et al. ER stress (PERK/eIF2α phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007;14(2):230–9.
Zhang X, **g Y, Qin C, Liu C, Yang D, Gao F, et al. Mechanical stress regulates autophagic flux to affect apoptosis after spinal cord injury. J Cell Mol Med. 2020;24(21):12765–76.
Li D, Lu Z, Xu Z, Ji J, Zheng Z, Lin S, et al. Spironolactone promotes autophagy via inhibiting PI3K/AKT/mTOR signalling pathway and reduce adhesive capacity damage in podocytes under mechanical stress. Biosci Rep. 2016;36(4):1.
Fu Y, Sun X, Lu B. HIPK3 modulates autophagy and HTT protein levels in neuronal and mouse models of Huntington disease. Autophagy. 2018;14(1):169–70.
Catanese A, Olde Heuvel F, Mulaw M, Demestre M, Higelin J, Barbi G, et al. Retinoic acid worsens ATG10-dependent autophagy impairment in TBK1-mutant hiPSC-derived motoneurons through SQSTM1/p62 accumulation. Autophagy. 2019;15(10):1719–37.
Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6(4):463–77.
Ashrafizadeh M, Paskeh MDA, Mirzaei S, Gholami MH, Zarrabi A, Hashemi F, et al. Targeting autophagy in prostate cancer: preclinical and clinical evidence for therapeutic response. J Exp Clin Cancer Res. 2022;41(1):1–37.
Paskeh MDA, Entezari M, Clark C, Zabolian A, Ranjbar E, Farahani MV, et al. Targeted regulation of autophagy using nanoparticles: new insight into cancer therapy. BBA Mol Basis Dis. 2022;1868(3):166326.
Wang X, Guo Z, Ding Z, Mehta JL. Inflammation, autophagy, and apoptosis after myocardial infarction. J Am Heart Assoc. 2018;7(9):1.
Liu CY, Zhang YH, Li RB, Zhou LY, An T, Zhang RC, et al. LncRNA CAIF inhibits autophagy and attenuates myocardial infarction by blocking p53-mediated myocardin transcription. Nat Commun. 2018;9(1):29.
Zare-Shahabadi A, Masliah E, Johnson GV, Rezaei N. Autophagy in Alzheimer’s disease. Rev Neurosci. 2015;26(4):385–95.
Rivero-Ríos P, Madero-Pérez J, Fernández B, Hilfiker S. Targeting the autophagy/lysosomal degradation pathway in Parkinson’s disease. Curr Neuropharmacol. 2016;14(3):238–49.
Kim J, Lim YM, Lee MS. The role of autophagy in systemic metabolism and human-type diabetes. Mol Cells. 2018;41(1):11–7.
Wei F, Wang D, Wei J, Tang N, Tang L, **ong F, et al. Metabolic crosstalk in the tumor microenvironment regulates antitumor immunosuppression and immunotherapy resisitance. Cell Mol Sci CMLS. 2021;78(1):173–93.
Yin H, Wu H, Chen Y, Zhang J, Zheng M, Chen G, et al. The therapeutic and pathogenic role of autophagy in autoimmune diseases. Front Immunol. 2018;9:1512.
Xu F, Yan W, Cheng Y. Pou4f3 gene mutation promotes autophagy and apoptosis of cochlear hair cells in cisplatin-induced deafness mice. Arch Biochem Biophys. 2020;680:108224.
Zhu HL, Xu XF, Shi XT, Feng YJ, **ong YW, Nan Y, et al. Activation of autophagy inhibits cadmium-triggered apoptosis in human placental trophoblasts and mouse placenta. Environ Pollut. 2019;254:112991.
Tan SC, Ankathil R. Genetic susceptibility to cervical cancer: role of common polymorphisms in apoptosis-related genes. Tumour Biol J Int Soc Oncodev Biol Med. 2015;36(9):6633–44.
Tan SC, Ismail MP, Duski DR, Othman NH. Ankathil R (2017) FAS c.-671A>G polymorphism and cervical cancer risk: a case-control study and meta-analysis. Cancer Genet. 2017;211:18–25.
Ren H, Zhao F, Zhang Q, Huang X, Wang Z. Autophagy and skin wound healing. Burns Trauma. 2022;10:tkac003.
Saha S, Panigrahi DP, Patil S, Bhutia SK. Autophagy in health and disease: a comprehensive review. Biomed Pharmacother. 2018;104:485–95.
Matsuzawa-Ishimoto Y, Hwang S, Cadwell K. Autophagy and inflammation. Annu Rev Immunol. 2018;36:73–101.
Runwal G, Stamatakou E, Siddiqi FH, Puri C, Zhu Y, Rubinsztein DC. LC3-positive structures are prominent in autophagy-deficient cells. Sci Rep. 2019;9(1):10147.
Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13(2):132–41.
Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221(1):3–12.
Peng X, Wei C, Li HZ, Li HX, Bai SZ, Wang LN, et al. NPS2390, a selective calcium-sensing receptor antagonist controls the phenotypic modulation of hypoxic human pulmonary arterial smooth muscle cells by regulating autophagy. J Transl Intern Med. 2019;7(2):59–68.
Tamargo-Gómez I, Mariño G. AMPK: regulation of metabolic dynamics in the context of autophagy. Int J Mol Sci. 2018;19(12):1.
Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18(4):571–80.
Zhang J, Ney PA. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 2009;16(7):939–46.
Qi J, **ng Y, Liu Y, Wang MM, Wei X, Sui Z, et al. MCOLN1/TRPML1 finely controls oncogenic autophagy in cancer by mediating zinc influx. Autophagy. 2021;17(12):4401–22.
Wang C, Zeng J, Li LJ, Xue M, He SL. Cdc25A inhibits autophagy-mediated ferroptosis by upregulating ErbB2 through PKM2 dephosphorylation in cervical cancer cells. Cell Death Dis. 2021;12(11):1055.
Miao CC, Hwang W, Chu LY, Yang LH, Ha CT, Chen PY, et al. LC3A-mediated autophagy regulates lung cancer cell plasticity. Autophagy. 2022;18(4):921–34.
Rotem-Dai N, Muraleedharan A, Livneh E. PKCeta promotes stress-induced autophagy and senescence in breast cancer cells, presenting a target for therapy. Pharmaceutics. 2022;14(8):1.
Yoo HS, Won SB, Kwon YH. Luteolin induces apoptosis and autophagy in HCT116 colon cancer cells via p53-dependent pathway. Nutr Cancer. 2022;74(2):677–86.
Weisheit S, Wegner CS, Ailte I, Radulovic M, Weyergang A, Selbo PK, et al. Inhibiting autophagy increases the efficacy of low-dose photodynamic therapy. Biochem Pharmacol. 2021;194:114837.
Togano S, Yashiro M, Masuda G, Sugimoto A, Miki Y, Yamamoto Y, et al. Gastric cancer stem cells survive in stress environments via their autophagy system. Sci Rep. 2021;11(1):20664.
Fan LX, Tao L, Lai YC, Cai SY, Zhao ZY, Yang F, et al. Cx32 promotes autophagy and produces resistance to SN-induced apoptosis via activation of AMPK signalling in cervical cancer. Int J Oncol. 2022;60(1):1.
Yang F, Peng ZX, Ji WD, Yu JD, Qian C, Liu JD, et al. LncRNA CCAT1 upregulates ATG5 to enhance autophagy and promote gastric cancer development by absorbing miR-140-3p. Dig Dis Sci. 2022;67(8):3725–41.
Najafi S, Tan SC, Raee P, Rahmati Y, Asemani Y, Lee EHC, et al. Gene regulation by antisense transcription: a focus on neurological and cancer diseases. Biomed Pharmacother. 2022;145:112265.
Wang CC, Lin SY, Huang YH, Hsieh CH, Chang HH, Chen HY, et al. Paired-like homeodomain 2B contributes to tumour progression and anti-autophagy in human lung cancer. Am J Cancer Res. 2021;11(10):4900–18.
Shu Y, Sun X, Ye G, Xu M, Wu Z, Wu C, et al. DHOK exerts anti-cancer effect through autophagy inhibition in colorectal cancer. Front Cell Dev Biol. 2021;9:760022.
Lan SH, Lin SC, Wang WC, Yang YC, Lee JC, Lin PW, et al. Autophagy upregulates miR-449a expression to suppress progression of colorectal cancer. Front Oncol. 2021;11:738144.
Tan SC. Low penetrance genetic polymorphisms as potential biomarkers for colorectal cancer predisposition. J Gene Med. 2018;20(4):e3010.
McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology (Baltimore, Md). 2021;73(Suppl 1):4–13.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Petrick JL, Florio AA, Znaor A, Ruggieri D, Laversanne M, Alvarez CS, et al. International trends in hepatocellular carcinoma incidence. Int J Cancer. 2020;147(2):317–30.
Valery PC, Laversanne M, Clark PJ, Petrick JL, McGlynn KA, Bray F. Projections of primary liver cancer to 2030 in 30 countries worldwide. Hepatology (Baltimore, MD). 2018;67(2):600–11.
Petrick JL, Florio AA, Loomba R, McGlynn KAJC. Have incidence rates of liver cancer peaked in the United States? Cancer. 2020;126(13):3151–5.
Zhang X, El-Serag HB, Thrift AP. Sex and race disparities in the incidence of hepatocellular carcinoma in the united states examined through age-period-cohort analysis. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2020;29(1):88–94.
Tsochatzis EA, Fatourou E, O’Beirne J, Meyer T, Burroughs AK. Transarterial chemoembolization and bland embolization for hepatocellular carcinoma. World J Gastroenterol. 2014;20(12):3069–77.
Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet (London, England). 2012;379(9822):1245–55.
Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58(3):593–608.
El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118–27.
Deldar Abad Paskeh M, Mirzaei S, Ashrafizadeh M, Zarrabi A, Sethi G. Wnt/β-catenin signaling as a driver of hepatocellular carcinoma progression: an emphasis on molecular pathways. 2021.
Yu Y, Peng XD, Qian XJ, Zhang KM, Huang X, Chen YH, et al. Fis1 phosphorylation by Met promotes mitochondrial fission and hepatocellular carcinoma metastasis. Signal Transduct Target Ther. 2021;6(1):401.
Zhang J, He X, Wan Y, Zhang H, Tang T, Zhang M, et al. CD44 promotes hepatocellular carcinoma progression via upregulation of YAP. Exp Hematol Oncol. 2021;10(1):54.
Becattini B, Breasson L, Sardi C, Zani F, Solinas G. PI3Kγ promotes obesity-associated hepatocellular carcinoma by regulating metabolism and inflammation. JHEP Rep Innov Hepatol. 2021;3(6):100359.
Li Q, Chao Q, Liu Y, Fang J, **e J, Zhen J, et al. Deubiquitinase ZRANB1 drives hepatocellular carcinoma progression through SP1-LOXL2 axis. Am J Cancer Res. 2021;11(10):4807–25.
Hu X, Yuan G, Li Q, Huang J, Cheng X, Chen J. DEAH-box polypeptide 32 promotes hepatocellular carcinoma progression via activating the β-catenin pathway. Ann Med. 2021;53(1):437–47.
Xu R, Yin S, Zheng M, Pei X, Ji X. Circular RNA circZFR promotes hepatocellular carcinoma progression by regulating miR-375/HMGA2 axis. Dig Dis Sci. 2021;66(12):4361–73.
Liu R, Li R, Yu H, Liu J, Zheng S, Li Y, et al. NTF3 correlates with prognosis and immune infiltration in hepatocellular carcinoma. Front Med. 2021;8:795849.
Pu J, Zhang Y, Wang A, Qin Z, Zhuo C, Li W, et al. ADORA2A-AS1 restricts hepatocellular carcinoma progression via binding HuR and repressing FSCN1/AKT Axis. Front Oncol. 2021;11:754835.
Kee HJ, Cheong JH. Tumor bioenergetics: an emerging avenue for cancer metabolism targeted therapy. BMB Rep. 2014;47(3):158–66.
Beyoğlu D, Imbeaud S, Maurhofer O, Bioulac-Sage P, Zucman-Rossi J, Dufour JF, et al. Tissue metabolomics of hepatocellular carcinoma: tumor energy metabolism and the role of transcriptomic classification. Hepatology (Baltimore, MD). 2013;58(1):229–38.
Li J, Hu ZQ, Yu SY, Mao L, Zhou ZJ, Wang PC, et al. CircRPN2 inhibits aerobic glycolysis and metastasis in hepatocellular carcinoma. Can Res. 2022;82(6):1055–69.
Wang Q, **e H, Peng H, Yan J, Han L, Ye G. ZC3H13 inhibits the progression of hepatocellular carcinoma through m(6)A-PKM2-mediated glycolysis and enhances chemosensitivity. J Oncol. 2021;2021:1328444.
Chang H, Li J, Luo Y, Wu B, Yuan C, Geng X. TFB2M activates aerobic glycolysis in hepatocellular carcinoma cells through the NAD(+) /SIRT3/HIF-1α signaling. J Gastroenterol Hepatol. 2021;36(10):2978–88.
Fan Q, Yang L, Zhang X, Ma Y, Li Y, Dong L, et al. Autophagy promotes metastasis and glycolysis by upregulating MCT1 expression and Wnt/β-catenin signaling pathway activation in hepatocellular carcinoma cells. J Exp Clin Cancer Res CR. 2018;37(1):9.
Liang H, **ong Z, Li R, Hu K, Cao M, Yang J, et al. BDH2 is downregulated in hepatocellular carcinoma and acts as a tumor suppressor regulating cell apoptosis and autophagy. J Cancer. 2019;10(16):3735–45.
Ashby K, Navarro Almario E, Tong W, Borlak J, Mehta R, Chen MJA, et al. Therapeutic bile acids and the risks for hepatotoxicity. Aliment Pharmacol Ther. 2018;47(12):1623–38.
Chen L, Han XJTJoci. Anti–PD-1/PD-L1 therapy of human cancer: past, present, and future. 2015;125(9):3384–91.
Gao Q, Wang X-Y, Qiu S-J, Yamato I, Sho M, Nakajima Y, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. J Clin Invest. 2009;15(3):971–9.
Kleffel S, Posch C, Barthel SR, Mueller H, Schlapbach C, Guenova E, et al. Melanoma cell-intrinsic PD-1 receptor functions promote tumor growth. Cell. 2015;162(6):1242–56.
Li H, Li X, Liu S, Guo L, Zhang B, Zhang J, et al. Programmed cell death-1 (PD-1) checkpoint blockade in combination with a mammalian target of rapamycin inhibitor restrains hepatocellular carcinoma growth induced by hepatoma cell–intrinsic PD-1. Hepatology. 2017;66(6):1920–33.
Gao QJAB. Diseases: oxidative stress and autophagy. Mini Rev Med Chem. 2019;22:179–98.
Bi Y, Jiang Y, Li X, Hou G. Oncology C: Rapamycin inhibits lung squamous cell carcinoma growth by downregulating glypican-3/Wnt/β-catenin signaling and autophagy. J Cancer Res Clin. 2021;147(2):499–505.
Wang CZ, Yan GX, Dong DS, **n H, Liu ZY. LncRNA-ATB promotes autophagy by activating Yes-associated protein and inducing autophagy-related protein 5 expression in hepatocellular carcinoma. World J Gastroenterol. 2019;25(35):5310–22.
Deust A, Chobert MN, Demontant V, Gricourt G, Denaës T, Thiolat A, et al. Macrophage autophagy protects against hepatocellular carcinogenesis in mice. Sci Rep. 2021;11(1):18809.
Wang F, Wu H, Zhang S, Lu J, Lu Y, Zhan P, et al. LAPTM4B facilitates tumor growth and induces autophagy in hepatocellular carcinoma. Cancer Manag Res. 2019;11:2485–97.
Wang Y, Zhao H, Wang D, Hao M, Kong C, Zhao X, et al. Inhibition of autophagy promoted apoptosis and suppressed growth of hepatocellular carcinoma upon photothermal exposure. J Biomed Nanotechnol. 2019;15(4):813–21.
Zhang H, Zhang Y, Zhu X, Chen C, Zhang C, **a Y, et al. DEAD box protein 5 inhibits liver tumorigenesis by stimulating autophagy via interaction with p62/SQSTM1. Hepatology (Baltimore, MD). 2019;69(3):1046–63.
Chen MY, Yadav VK, Chu YC, Ong JR, Huang TY, Lee KF, et al. Hydroxychloroquine (HCQ) modulates autophagy and oxidative DNA damage stress in hepatocellular carcinoma to overcome sorafenib resistance via TLR9/SOD1/hsa-miR-30a-5p/Beclin-1 axis. Cancers. 2021;13(13):1.
Zhang YJ, Pan Q, Yu Y, Zhong XP. microRNA-519d induces autophagy and apoptosis of human hepatocellular carcinoma cells through activation of the AMPK signaling pathway via Rab10. Cancer Manag Res. 2020;12:2589–602.
Zhang T, Yang X, Xu W, Wang J, Wu D, Hong Z, et al. Heat shock protein 90 promotes RNA helicase DDX5 accumulation and exacerbates hepatocellular carcinoma by inhibiting autophagy. Cancer Biol Med. 2021;18(3):693–704.
Wang LL, ** XH, Cai MY, Li HG, Chen JW, Wang FW, et al. AGBL2 promotes cancer cell growth through IRGM-regulated autophagy and enhanced Aurora A activity in hepatocellular carcinoma. Cancer Lett. 2018;414:71–80.
Hu P, Ke C, Guo X, Ren P, Tong Y, Luo S, et al. Both glypican-3/Wnt/β-catenin signaling pathway and autophagy contributed to the inhibitory effect of curcumin on hepatocellular carcinoma. Digest Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver. 2019;51(1):120–6.
Sorice M. Crosstalk of autophagy and apoptosis. Cells. 2022;11(9):1.
Han B, Wang Y, Zheng M. Inhibition of autophagy promotes human RSV NS1-induced inflammation and apoptosis in vitro. Exp Ther Med. 2021;22(4):1054.
Yang J, Sun Y, Xu F, Liu W, Hayashi T, Mizuno K, et al. Autophagy and glycolysis independently attenuate silibinin-induced apoptosis in human hepatocarcinoma HepG2 and Hep3B cells. Hum Exp Toxicol. 2021;40(12):2048–62.
Shan Y, Li P. Long intergenic non-protein coding RNA 665 regulates viability, apoptosis, and autophagy via the MiR-186-5p/MAP4K3 Axis in hepatocellular carcinoma. Yonsei Med J. 2019;60(9):842–53.
Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science (New York, NY). 2016;352(6282):175–80.
Wang X, Li Y, Li Z, Lin S, Wang H, Sun J, et al. Mitochondrial calcium uniporter drives metastasis and confers a targetable cystine dependency in pancreatic cancer. Can Res. 2022;82(12):2254–68.
Roy S, Banerjee P, Ekser B, Bayless K, Zawieja D, Alpini G, et al. Targeting lymphangiogenesis and lymph node metastasis in liver cancer. Am J Pathol. 2021;191(12):2052–63.
Deng L, Wang D, Chen S, Hu W, Zhang R. Epiphycan predicts poor outcomes and promotes metastasis in ovarian cancer. Front Oncol. 2021;11:653782.
Lei RE, Shi C, Zhang PL, Hu BL, Jiang HX, Qin SY. IL-9 promotes proliferation and metastasis of hepatocellular cancer cells by activating JAK2/STAT3 pathway. Int J Clin Exp Pathol. 2017;10(7):7940–6.
Yang L, Qiu J, **ao Y, Hu X, Liu Q, Chen L, et al. AP-2β inhibits hepatocellular carcinoma invasion and metastasis through Slug and Snail to suppress epithelial-mesenchymal transition. Theranostics. 2018;8(13):3707–21.
Ning S, Bin C, Na H, Peng S, Yi D, **ang-hua Y, et al. Glypican-3, a novel prognostic marker of hepatocellular cancer, is related with postoperative metastasis and recurrence in hepatocellular cancer patients. Mol Biol Rep. 2012;39(1):351–7.
Wu Q, Zhou W, Yin S, Zhou Y, Chen T, Qian J, et al. Blocking triggering receptor expressed on myeloid cells-1-positive tumor-associated macrophages induced by hypoxia reverses immunosuppression and anti-programmed cell death ligand 1 resistance in liver cancer. Hepatology (Baltimore, MD). 2019;70(1):198–214.
Amaravadi RK, Kimmelman AC, Debnath J. Targeting autophagy in cancer: recent advances and future directions. Cancer Discov. 2019;9(9):1167–81.
Kirkin V, Rogov VV. A diversity of selective autophagy receptors determines the specificity of the autophagy pathway. Mol Cell. 2019;76(2):268–85.
Chang Y, Yan W, He X, Zhang L, Li C, Huang H, et al. miR-375 inhibits autophagy and reduces viability of hepatocellular carcinoma cells under hypoxic conditions. Gastroenterology. 2012;143(1):177-87.e8.
Xu Z, Li Z, Wang W, **a Y, He Z, Li B, et al. MIR-1265 regulates cellular proliferation and apoptosis by targeting calcium binding protein 39 in gastric cancer and thereby, impairing oncogenic autophagy. Cancer Lett. 2019;449:226–36.
Liu S, Lin H, Wang D, Li Q, Luo H, Li G, et al. PCDH17 increases the sensitivity of colorectal cancer to 5-fluorouracil treatment by inducing apoptosis and autophagic cell death. Signal Transduct Target Ther. 2019;4:53.
Li Q, Ni Y, Zhang L, Jiang R, Xu J, Yang H, et al. HIF-1α-induced expression of m6A reader YTHDF1 drives hypoxia-induced autophagy and malignancy of hepatocellular carcinoma by promoting ATG2A and ATG14 translation. Signal Transduct Target Ther. 2021;6(1):76.
Endo TA, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, et al. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387(6636):921–4.
Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, et al. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387(6636):917–21.
Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol. 2004;22:503–29.
Yoon S, Yi YS, Kim SS, Kim JH, Park WS, Nam SW. SOCS5 and SOCS6 have similar expression patterns in normal and cancer tissues. Tumour Biol J Int Soc Oncodev Biol Med. 2012;33(1):215–21.
Yuan J, Li Y, Liao J, Liu M, Zhu L, Liao K. MicroRNA-7 inhibits hepatocellular carcinoma cell invasion and metastasis by regulating Atg5-mediated autophagy. Transl Cancer Res. 2020;9(6):3965–72.
Ling H, Spizzo R, Atlasi Y, Nicoloso M, Shimizu M, Redis RS, et al. CCAT2, a novel noncoding RNA map** to 8q24, underlies metastatic progression and chromosomal instability in colon cancer. Genome Res. 2013;23(9):1446–61.
Xu Y, Wang B, Zhang F, Wang A, Du X, Hu P, et al. Long non-coding RNA CCAT2 is associated with poor prognosis in hepatocellular carcinoma and promotes tumor metastasis by regulating Snail2-mediated epithelial-mesenchymal transition. Onco Targets Ther. 2017;10:1191–8.
Liu Y, Wang D, Li Y, Yan S, Dang H, Yue H, et al. Long noncoding RNA CCAT2 promotes hepatocellular carcinoma proliferation and metastasis through up-regulation of NDRG1. Exp Cell Res. 2019;379(1):19–29.
Niu C, Wang L, Ye W, Guo S, Bao X, Wang Y, et al. CCAT2 contributes to hepatocellular carcinoma progression via inhibiting miR-145 maturation to induce MDM2 expression. J Cell Physiol. 2020;235(9):6307–20.
Shi J, Guo C, Ma J. CCAT2 enhances autophagy-related invasion and metastasis via regulating miR-4496 and ELAVL1 in hepatocellular carcinoma. J Cell Mol Med. 2021;25(18):8985–96.
Li X, Gao L, Zheng L, Shi J, Ma J. BMP4-mediated autophagy is involved in the metastasis of hepatocellular carcinoma via JNK/Beclin1 signaling. Am J Transl Res. 2020;12(6):3068–77.
Bucher BT, Feng X, Jeyabalan G, Zhang B, Shao L, Guo Z, et al. Glycochenodeoxycholate (GCDC) inhibits cytokine induced iNOS expression in rat hepatocytes. J Surg Res. 2007;138(1):15–21.
Chiang JYJC. Bile acid metabolism and signaling. Comprehen Physiol. 2013;3(3):1191.
Allen K, Jaeschke H. Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am J Pathol. 2011;178(1):175–86.
Fickert P, Wagner MJJ. Biliary bile acids in hepatobiliary injury—what is the link? J Hepatol. 2017;67(3):619–31.
Graf D, Kohlmann C, Haselow K, Gehrmann T, Bode JG, Häussinger DJH. Bile acids inhibit interleukin-6 signaling via gp130 receptor-dependent and-independent pathways in rat liver. Hepatology. 2006;44(5):1206–17.
Wang K, Brems JJ, Gamelli RL, Ding J. Reversibility of caspase activation and its role during glycochenodeoxycholate-induced hepatocyte apoptosis. J Biol Chem. 2005;280(25):23490–5.
Gao L, Lv G, Li R, Liu WT, Zong C, Ye F, et al. Glycochenodeoxycholate promotes hepatocellular carcinoma invasion and migration by AMPK/mTOR dependent autophagy activation. Cancer Lett. 2019;454:215–23.
Liu WR, ** L, Tian MX, Jiang XF, Yang LX, Ding ZB, et al. Caveolin-1 promotes tumor growth and metastasis via autophagy inhibition in hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2016;40(2):169–78.
Nieto MA, Huang RYJ, Jackson RA, Thiery JPJC. EMT. ACS Appl Bio Mater. 2016;166(1):21–45.
Ashrafizadeh M, Zarrabi A, Hushmandi K, Kalantari M, Mohammadinejad R, Javaheri T, et al. Association of the epithelial–mesenchymal transition (EMT) with cisplatin resistance. Int J Mol Sci. 2020;21(11):4002.
Ashrafizadeh M, Mirzaei S, Hashemi F, Zarrabi A, Zabolian A, Saleki H, et al. New insight towards development of paclitaxel and docetaxel resistance in cancer cells: EMT as a novel molecular mechanism and therapeutic possibilities. Biomed Pharmacother. 2021;141:111824.
Ashrafizadeh M, Hushmandi K, Hashemi M, Akbari ME, Kubatka P, Raei M, et al. Role of microRNA/epithelial-to-mesenchymal transition axis in the metastasis of bladder cancer. Biomolecules. 2020;10(8):1159.
Su G, Feng T, Pei T, Yang F, Sun D, Yu H, et al. Autophagy modulates FSS-induced epithelial-mesenchymal transition in hepatocellular carcinoma cells. Mol Carcinog. 2021;60(9):607–19.
Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13(5):555–62.
Simpson CD, Anyiwe K, Schimmer AD. Anoikis resistance and tumor metastasis. Cancer Lett. 2008;272(2):177–85.
Kroemer G, Piacentini M. Dying to survive—apoptosis, necroptosis, autophagy as supreme experiments of nature. Int J Dev Biol. 2015;59(1–3):5–9.
Wang S, Lv Y, Zhou Y, Ling J, Wang H, Gu D, et al. Acidic extracellular pH induces autophagy to promote anoikis resistance of hepatocellular carcinoma cells via downregulation of miR-3663-3p. J Cancer. 2021;12(12):3418–26.
Fu XT, Shi YH, Zhou J, Peng YF, Liu WR, Shi GM, et al. MicroRNA-30a suppresses autophagy-mediated anoikis resistance and metastasis in hepatocellular carcinoma. Cancer Lett. 2018;412:108–17.
Zhu HD, Liu L, Deng H, Li ZB, Sheng JQ, He XX, et al. Astrocyte elevated gene 1 (AEG-1) promotes anoikis resistance and metastasis by inducing autophagy in hepatocellular carcinoma. J Cell Physiol. 2020;235(6):5084–95.
Qiao L, Zhang Q, Sun Z, Liu Q, Wu Z, Hu W, et al. The E2F1/USP11 positive feedback loop promotes hepatocellular carcinoma metastasis and inhibits autophagy by activating ERK/mTOR pathway. Cancer Lett. 2021;514:63–78.
Sun Y, Xu H, Chen X, Li X, Luo B. Inhibition of mitochondrial respiration overcomes hepatocellular carcinoma chemoresistance. Biochem Biophys Res Commun. 2019;508(2):626–32.
Xu J, Zhu Y, Wang F, Zhou Y, **a G, Xu W. ICMT contributes to hepatocellular carcinoma growth, survival, migration and chemoresistance via multiple oncogenic pathways. Biochem Biophys Res Commun. 2019;518(3):584–9.
Cai L, ** X, Zhang J, Li L, Zhao J. Metformin suppresses Nrf2-mediated chemoresistance in hepatocellular carcinoma cells by increasing glycolysis. Aging. 2020;12(17):17582–600.
Zhang W, Zhang J, Xu C, Zhang S, Bian S, Jiang F, et al. Ubiquitin-specific protease 7 is a drug-able target that promotes hepatocellular carcinoma and chemoresistance. Cancer Cell Int. 2020;20:28.
Shi C, Yang J, Hu L, Liao B, Qiao L, Shen W, et al. Glycochenodeoxycholic acid induces stemness and chemoresistance via the STAT3 signaling pathway in hepatocellular carcinoma cells. Aging. 2020;12(15):15546–55.
Lu S, Yao Y, Xu G, Zhou C, Zhang Y, Sun J, et al. CD24 regulates sorafenib resistance via activating autophagy in hepatocellular carcinoma. Cell Death Dis. 2018;9(6):646.
Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12(12):931–47.
Kudo Y, Sugimoto M, Arias E, Kasashima H, Cordes T, Linares JF, et al. PKCλ/ι loss induces autophagy, oxidative phosphorylation, and NRF2 to promote liver cancer progression. Cancer Cell. 2020;38(2):247-62.e11.
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–72.
Louandre C, Ezzoukhry Z, Godin C, Barbare JC, Mazière JC, Chauffert B, et al. Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int J Cancer. 2013;133(7):1732–42.
Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Life. 2014;3:e02523.
Li B, Wei S, Yang L, Peng X, Ma Y, Wu B, et al. CISD2 promotes resistance to sorafenib-induced ferroptosis by regulating autophagy in hepatocellular carcinoma. Front Oncol. 2021;11:657723.
Xu WP, Liu JP, Feng JF, Zhu CP, Yang Y, Zhou WP, et al. miR-541 potentiates the response of human hepatocellular carcinoma to sorafenib treatment by inhibiting autophagy. Gut. 2020;69(7):1309–21.
Feng X, Zou B, Nan T, Zheng X, Zheng L, Lan J, et al. MiR-25 enhances autophagy and promotes sorafenib resistance of hepatocellular carcinoma via targeting FBXW7. Int J Med Sci. 2022;19(2):257–66.
Bhawal UK, Ozaki Y, Nishimura M, Sugiyama M, Sasahira T, Nomura Y, et al. Association of expression of receptor for advanced glycation end products and invasive activity of oral squamous cell carcinoma. Oncology. 2005;69(3):246–55.
Yan SF, Ramasamy R, Schmidt AM. Receptor for AGE (RAGE) and its ligands-cast into leading roles in diabetes and the inflammatory response. J Mol Med (Berl). 2009;87(3):235–47.
Yaser AM, Huang Y, Zhou RR, Hu GS, **ao MF, Huang ZB, et al. The role of receptor for advanced glycation end products (RAGE) in the proliferation of hepatocellular carcinoma. Int J Mol Sci. 2012;13(5):5982–97.
Li S, Gu H, Huang Y, Peng Q, Zhou R, Yi P, et al. Circular RNA 101368/miR-200a axis modulates the migration of hepatocellular carcinoma through HMGB1/RAGE signaling. Cell Cycle (Georgetown, TX). 2018;17(19–20):2349–59.
Li J, Wu PW, Zhou Y, Dai B, Zhang PF, Zhang YH, et al. Rage induces hepatocellular carcinoma proliferation and sorafenib resistance by modulating autophagy. Cell Death Dis. 2018;9(2):225.
** Y, Yang R, Ding J, Zhu F, Zhu C, Xu Q, et al. KAT6A is associated with sorafenib resistance and contributes to progression of hepatocellular carcinoma by targeting YAP. Biochem Biophys Res Commun. 2021;585:185–90.
Zheng L, Du J, Ge F, Qian M, Yang B, He Q, et al. The calcimimetic agent cinacalcet inhibits hepatocellular carcinoma via YAP/TAZ suppression. Pharmazie. 2021;76(10):511–4.
Yu H, He J, Su G, Wang Y, Fang F, Yang W, et al. Fluid shear stress activates YAP to promote epithelial-mesenchymal transition in hepatocellular carcinoma. Mol Oncol. 2021;15(11):3164–83.
Zhou Y, Wang Y, Zhou W, Chen T, Wu Q, Chutturghoon VK, et al. YAP promotes multi-drug resistance and inhibits autophagy-related cell death in hepatocellular carcinoma via the RAC1-ROS-mTOR pathway. Cancer Cell Int. 2019;19:179.
Boyce M, Yuan JJCD. Differentiation. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ. 2006;13(3):363–73.
Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Investig. 2005;115(4):951–8.
Ashrafizadeh M, Tavakol S, Ahmadi Z, Roomiani S, Mohammadinejad R, Samarghandian S. Therapeutic effects of kaempferol affecting autophagy and endoplasmic reticulum stress. Phytother Res. 2020;34(5):911–23.
Shi YH, Ding ZB, Zhou J, Hui B, Shi GM, Ke AW, et al. Targeting autophagy enhances sorafenib lethality for hepatocellular carcinoma via ER stress-related apoptosis. Autophagy. 2011;7(10):1159–72.
Fu XT, Song K, Zhou J, Shi YH, Liu WR, Tian MX, et al. Autophagy activation contributes to glutathione transferase Mu 1-mediated chemoresistance in hepatocellular carcinoma. Oncol Lett. 2018;16(1):346–52.
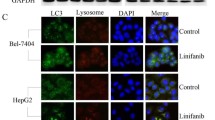
Pan H, Wang Z, Jiang L, Sui X, You L, Shou J, et al. Autophagy inhibition sensitizes hepatocellular carcinoma to the multikinase inhibitor linifanib. Sci Rep. 2014;4:6683.
Cheng CC, Ho AS, Peng CL, Chang J, Sie ZL, Wang CL, et al. Sorafenib suppresses radioresistance and synergizes radiotherapy-mediated CD8(+) T cell activation to eradicate hepatocellular carcinoma. Int Immunopharmacol. 2022;112:109110.
Lin LC, Chen CF, Ho CT, Liu JJ, Liu TZ, Chern CL. γ-Glutamylcysteine synthetase (γ-GCS) as a target for overcoming chemo- and radio-resistance of human hepatocellular carcinoma cells. Life Sci. 2018;198:25–31.
Wang M, Cheng H, Wu H, Liu C, Li S, Li B, et al. Gambogenic acid antagonizes the expression and effects of long non-coding RNA NEAT1 and triggers autophagy and ferroptosis in melanoma. Biomed Pharmacother. 2022;154:113636.
Sakaguchi H, Tsuchiya H, Kitagawa Y, Tanino T, Yoshida K, Uchida N, et al. NEAT1 confers radioresistance to hepatocellular carcinoma cells by inducing autophagy through GABARAP. Int J Mol Sci. 2022;23(2):1.
Altmeyer A, Jung AC, Ignat M, Benzina S, Denis JM, Gueulette J, et al. Pharmacological enhancement of autophagy induced in a hepatocellular carcinoma cell line by high-LET radiation. Anticancer Res. 2010;30(2):303–10.
Trigiante G, Lu X. ASPP [corrected] and cancer. Nat Rev Cancer. 2006;6(3):217–26.
Zhao J, Wu G, Bu F, Lu B, Liang A, Cao L, et al. Epigenetic silence of ankyrin-repeat-containing, SH3-domain-containing, and proline-rich-region- containing protein 1 (ASPP1) and ASPP2 genes promotes tumor growth in hepatitis B virus-positive hepatocellular carcinoma. Hepatology (Baltimore, MD). 2010;51(1):142–53.
Wang Y, Wang XD, Lapi E, Sullivan A, Jia W, He YW, et al. Autophagic activity dictates the cellular response to oncogenic RAS. Proc Natl Acad Sci USA. 2012;109(33):13325–30.
Chen R, Wang H, Liang B, Liu G, Tang M, Jia R, et al. Downregulation of ASPP2 improves hepatocellular carcinoma cells survival via promoting BECN1-dependent autophagy initiation. Cell Death Dis. 2016;7(12):e2512.
Altmeyer A, Josset E, Denis JM, Gueulette J, Slabbert J, Mutter D, et al. The mTOR inhibitor RAD001 augments radiation-induced growth inhibition in a hepatocellular carcinoma cell line by increasing autophagy. Int J Oncol. 2012;41(4):1381–6.
Tian YD, Lin S, Yang PT, Bai MH, ** YY, Min WL, et al. Saikosaponin-d increases the radiosensitivity of hepatoma cells by adjusting cell autophagy. J Cancer. 2019;10(20):4947–53.
Claro S, Oshiro ME, Mortara RA, Paredes-Gamero EJ, Pereira GJ, Smaili SS, et al. γ-Rays-generated ROS induce apoptosis via mitochondrial and cell cycle alteration in smooth muscle cells. Int J Radiat Biol. 2014;90(10):914–27.
Hoorelbeke D, Decrock E, De Smet M, De Bock M, Descamps B, Van Haver V, et al. Cx43 channels and signaling via IP(3)/Ca(2+), ATP, and ROS/NO propagate radiation-induced DNA damage to non-irradiated brain microvascular endothelial cells. Cell Death Dis. 2020;11(3):194.
Xu Y, Ji Y, Li X, Ding J, Chen L, Huang Y, et al. URI1 suppresses irradiation-induced reactive oxygen species (ROS) by activating autophagy in hepatocellular carcinoma cells. Int J Biol Sci. 2021;17(12):3091–103.
Peng WX, Wan YY, Gong AH, Ge L, ** J, Xu M, et al. Egr-1 regulates irradiation-induced autophagy through Atg4B to promote radioresistance in hepatocellular carcinoma cells. Oncogenesis. 2017;6(1):e292.
Abdel-Rafei MK, Thabet NM, Rashed LA, Moustafa EM. Canagliflozin, a SGLT-2 inhibitor, relieves ER stress, modulates autophagy and induces apoptosis in irradiated HepG2 cells: Signal transduction between PI3K/AKT/GSK-3β/mTOR and Wnt/β-catenin pathways; in vitro. J Cancer Res Ther. 2021;17(6):1404–18.
Choi C, Son A, Lee HS, Lee YJ, Park HC. Radiosensitization by marine Sponge Agelas sp. extracts in hepatocellular carcinoma cells with autophagy induction. Sci Rep. 2018;8(1):6317.
Lin J, Ruan J, Zhu H, Chen Z, Chen J, Yu H. Tenacissoside H induces autophagy and radiosensitivity of hepatocellular carcinoma cells by PI3K/Akt/mTOR signaling pathway. Dose Response Publ Int Hormesis Soc. 2021;19(2):15593258211011024.
Zhu L, Zhao Y, Yu L, He X, Wang Y, Jiang P, et al. Overexpression of ADAM9 decreases radiosensitivity of hepatocellular carcinoma cell by activating autophagy. Bioengineered. 2021;12(1):5516–28.
Wei CY, Zhu MX, Zhang PF, Huang XY, Wan JK, Yao XZ, et al. PKCα/ZFP64/CSF1 axis resets the tumor microenvironment and fuels anti-PD1 resistance in hepatocellular carcinoma. J Hepatol. 2022;77(1):163–76.
Lu Y, Shen H, Huang W, He S, Chen J, Zhang D, et al. Genome-scale CRISPR-Cas9 knockout screening in hepatocellular carcinoma with lenvatinib resistance. Cell Death Discov. 2021;7(1):359.
Huang W, Chen K, Lu Y, Zhang D, Cheng Y, Li L, et al. ABCC5 facilitates the acquired resistance of sorafenib through the inhibition of SLC7A11-induced ferroptosis in hepatocellular carcinoma. Neoplasia (New York, NY). 2021;23(12):1227–39.
Zhang J, Shang L, Jiang W, Wu W. Shikonin induces apoptosis and autophagy via downregulation of pyrroline-5-carboxylate reductase1 in hepatocellular carcinoma cells. Bioengineered. 2022;13(3):7904–18.
Ji A, Hu L, Ma D, Qiang G, Yan D, Zhang G, et al. Myricetin induces apoptosis and protective autophagy through endoplasmic reticulum stress in hepatocellular carcinoma. Evid Based Complem Alternat Med eCAM. 2022;2022:3115312.
Keshavarz-Pakseresht B, Shandiz SA, Baghbani-Arani F. Imatinib induces up-regulation of NM23, a metastasis suppressor gene, in human Hepatocarcinoma (HepG2) Cell Line. Gastroenterol Hepatol Bed Bench. 2017;10(1):29–33.
Shamaa MM. Sulfasalazine synergistically enhances the inhibitory effects of imatinib against hepatocellular carcinoma (HCC) cells by targeting NFκB, BCR/ABL, and PI3K/AKT signaling pathway-related proteins. FEBS Open Bio. 2021;11(3):588–97.
Zhang H, Yi J, Yoon D, Ryoo Z, Lee I, Kim M. Imatinib and GNF-5 exhibit an inhibitory effect on growth of hepatocellar carcinoma cells by downregulating S-phase kinase-associated protein 2. J Cancer Prev. 2020;25(4):252–7.
Nisha R, Kumar P, Kumar U, Mishra N, Maurya P, Singh S, et al. Fabrication of imatinib mesylate-loaded lactoferrin-modified pegylated liquid crystalline nanoparticles for mitochondrial-dependent apoptosis in hepatocellular carcinoma. Mol Pharm. 2021;18(3):1102–20.
**ao MC, Qian H, Huang CK, Zheng BN, Yan FZ, Liu F, et al. Imatinib inhibits the malignancy of hepatocellular carcinoma by suppressing autophagy. Eur J Pharmacol. 2021;906:174217.
Chen B, Zhou S, Zhan Y, Ke J, Wang K, Liang Q, et al. Dioscin inhibits the invasion and migration of hepatocellular carcinoma HepG2 cells by reversing TGF-β1-induced epithelial-mesenchymal transition. Molecules (Basel, Switzerland). 2019;24(12):1.
Zhang G, Zeng X, Zhang R, Liu J, Zhang W, Zhao Y, et al. Dioscin suppresses hepatocellular carcinoma tumor growth by inducing apoptosis and regulation of TP53, BAX, BCL2 and cleaved CASP3. Phytomed Int J Phytother Phytopharmacol. 2016;23(12):1329–36.
Mao Z, Han X, Chen D, Xu Y, Xu L, Yin L, et al. Potent effects of dioscin against hepatocellular carcinoma through regulating TP53-induced glycolysis and apoptosis regulator (TIGAR)-mediated apoptosis, autophagy, and DNA damage. Br J Pharmacol. 2019;176(7):919–37.
Okubo S, Ohta T, Fujita H, Shoyama Y, Uto T. Costunolide and dehydrocostuslactone from Saussurea lappa root inhibit autophagy in hepatocellular carcinoma cells. J Nat Med. 2021;75(1):240–5.
Shui L, Wang W, **e M, Ye B, Li X, Liu Y, et al. Isoquercitrin induces apoptosis and autophagy in hepatocellular carcinoma cells via AMPK/mTOR/p70S6K signaling pathway. Aging. 2020;12(23):24318–32.
Wang F, Qin C, Li Y, Qu W, Liu H, Li B, et al. Ursodeoxycholic acid induces autophagy via LC3B to suppress hepatocellular carcinoma in vivo and in vitro. Int J Clin Exp Pathol. 2017;10(12):11805–13.
Dai H, Li M, Yang W, Sun X, Wang P, Wang X, et al. Resveratrol inhibits the malignant progression of hepatocellular carcinoma via MARCH1-induced regulation of PTEN/AKT signaling. Aging. 2020;12(12):11717–31.
Zhang Q, Huang H, Zheng F, Liu H, Qiu F, Chen Y, et al. Resveratrol exerts antitumor effects by downregulating CD8(+)CD122(+) Tregs in murine hepatocellular carcinoma. Oncoimmunology. 2020;9(1):1829346.
Yan Y, Zhou C, Li J, Chen K, Wang G, Wei G, et al. Resveratrol inhibits hepatocellular carcinoma progression driven by hepatic stellate cells by targeting Gli-1. Mol Cell Biochem. 2017;434(1–2):17–24.
Zhang B, Yin X, Sui S. Resveratrol inhibited the progression of human hepatocellular carcinoma by inducing autophagy via regulating p53 and the phosphoinositide 3-kinase/protein kinase B pathway. Oncol Rep. 2018;40(5):2758–65.
Chen SY, Chao CN, Huang HY, Fang CY. Flavopereirine inhibits hepatocellular carcinoma cell growth by inducing cell-cycle arrest, apoptosis, and autophagy-related protein expression. Anticancer Res. 2020;40(12):6907–14.
Dawood M, Hegazy MF, Elbadawi M, Fleischer E, Klinger A, Bringmann G, et al. Vitamin K(3) chloro derivative (VKT-2) inhibits HDAC6, activates autophagy and apoptosis, and inhibits aggresome formation in hepatocellular carcinoma cells. Biochem Pharmacol. 2020;180:114176.
Liu S, Zhang J, Yang H, Zhang Q, Chen M. Pectolinarigenin flavonoid exhibits selective anti-proliferative activity in cisplatin-resistant hepatocellular carcinoma, autophagy activation, inhibiting cell migration and invasion, G2/M phase cell cycle arrest and targeting ERK1/2 MAP kinases. J BUON Official J Balkan Union Oncol. 2020;25(1):415–20.
Zhu L, Wu Q, Quan B, Yang J, Yang J, Hou W, et al. Autophagy inhibition by reversine and its suppressive effects on human hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2020;528(1):105–11.
Moballegh Nasery M, Abadi B, Poormoghadam D, Zarrabi A, Keyhanvar P, Khanbabaei H, et al. Curcumin delivery mediated by bio-based nanoparticles: a review. Molecules. 2020;25(3):689.
Ahmadi Z, Mohammadinejad R. Drug delivery systems for resveratrol, a non-flavonoid polyphenol: emerging evidence in last decades. J Drug Deliv Sci Technol. 2019;51:591–604.
Niture S, Gyamfi MA, Lin M, Chimeh U, Dong X, Zheng W, et al. TNFAIP8 regulates autophagy, cell steatosis, and promotes hepatocellular carcinoma cell proliferation. Cell Death Dis. 2020;11(3):178.
Yang J, Pi C, Wang G. Inhibition of PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy in hepatocellular carcinoma cells. Biomed Pharmacother. 2018;103:699–707.
Sheng JQ, Wang MR, Fang D, Liu L, Huang WJ, Tian DA, et al. LncRNA NBR2 inhibits tumorigenesis by regulating autophagy in hepatocellular carcinoma. Biomed Pharmacother. 2021;133:111023.
Han Z, Liu D, Chen L, He Y, Tian X, Qi L, et al. PNO1 regulates autophagy and apoptosis of hepatocellular carcinoma via the MAPK signaling pathway. Cell Death Dis. 2021;12(6):552.
Zhou M, Zhang G, Hu J, Zhu Y, Lan H, Shen X, et al. Rutin attenuates sorafenib-induced chemoresistance and autophagy in hepatocellular carcinoma by regulating BANCR/miRNA-590-5P/OLR1 Axis. Int J Biol Sci. 2021;17(13):3595–607.
An Y, Jiang J, Zhou L, Shi J, ** P, Li L, et al. Peroxiredoxin 1 is essential for natamycin-triggered apoptosis and protective autophagy in hepatocellular carcinoma. Cancer Lett. 2021;521:210–23.
Zhang X, ** L, Tian Z, Wang J, Yang Y, Liu J, et al. Nitric oxide inhibits autophagy and promotes apoptosis in hepatocellular carcinoma. Cancer Sci. 2019;110(3):1054–63.
Zheng J, Shao Y, Jiang Y, Chen F, Liu S, Yu N, et al. Tangeretin inhibits hepatocellular carcinoma proliferation and migration by promoting autophagy-related BECLIN1. Cancer Manag Res. 2019;11:5231–42.
Xue L, Liu P. Daurisoline inhibits hepatocellular carcinoma progression by restraining autophagy and promoting cispaltin-induced cell death. Biochem Biophys Res Commun. 2021;534:1083–90.
Cui J, Liu J, Fan L, Zhu Y, Zhou B, Wang Y, et al. A zinc finger family protein, ZNF263, promotes hepatocellular carcinoma resistance to apoptosis via activation of ER stress-dependent autophagy. Transl Oncol. 2020;13(12):100851.
Che N, Ng KY, Wong TL, Tong M, Kau PW, Chan LH, et al. PRMT6 deficiency induces autophagy in hostile microenvironments of hepatocellular carcinoma tumors by regulating BAG5-associated HSC70 stability. Cancer Lett. 2021;501:247–62.
Tong Y, Huang H, Pan H. Inhibition of MEK/ERK activation attenuates autophagy and potentiates pemetrexed-induced activity against HepG2 hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2015;456(1):86–91.
Lin Z, Niu Y, Wan A, Chen D, Liang H, Chen X, et al. RNA m(6) A methylation regulates sorafenib resistance in liver cancer through FOXO3-mediated autophagy. EMBO J. 2020;39(12):e103181.
Huang X, Gan G, Wang X, Xu T, **e W. The HGF-MET axis coordinates liver cancer metabolism and autophagy for chemotherapeutic resistance. Autophagy. 2019;15(7):1258–79.
Liang C, Dong Z, Cai X, Shen J, Xu Y, Zhang M, et al. Hypoxia induces sorafenib resistance mediated by autophagy via activating FOXO3a in hepatocellular carcinoma. Cell Death Dis. 2020;11(11):1017.
Li J, Zhou W, Mao Q, Gao D, **ong L, Hu X, et al. HMGB1 promotes resistance to doxorubicin in human hepatocellular carcinoma cells by inducing autophagy via the AMPK/mTOR signaling pathway. Front Oncol. 2021;11:739145.
Liu G, Fan X, Tang M, Chen R, Wang H, Jia R, et al. Osteopontin induces autophagy to promote chemo-resistance in human hepatocellular carcinoma cells. Cancer Lett. 2016;383(2):171–82.
Fu XT, Song K, Zhou J, Shi YH, Liu WR, Shi GM, et al. Tumor-associated macrophages modulate resistance to oxaliplatin via inducing autophagy in hepatocellular carcinoma. Cancer Cell Int. 2019;19:71.
Chen E, Li E, Liu H, Zhou Y, Wen L, Wang J, et al. miR-26b enhances the sensitivity of hepatocellular carcinoma to Doxorubicin via USP9X-dependent degradation of p53 and regulation of autophagy. Int J Biol Sci. 2021;17(3):781–95.
**g Z, Ye X, Ma X, Hu X, Yang W, Shi J, et al. SNGH16 regulates cell autophagy to promote Sorafenib Resistance through suppressing miR-23b-3p via sponging EGR1 in hepatocellular carcinoma. Cancer Med. 2020;9(12):4324–38.
Peng WX, **ong EM, Ge L, Wan YY, Zhang CL, Du FY, et al. Egr-1 promotes hypoxia-induced autophagy to enhance chemo-resistance of hepatocellular carcinoma cells. Exp Cell Res. 2016;340(1):62–70.
He C, Dong X, Zhai B, Jiang X, Dong D, Li B, et al. MiR-21 mediates sorafenib resistance of hepatocellular carcinoma cells by inhibiting autophagy via the PTEN/Akt pathway. Oncotarget. 2015;6(30):28867–81.
Liu D, Fan Y, Li J, Cheng B, Lin W, Li X, et al. Inhibition of cFLIP overcomes acquired resistance to sorafenib via reducing ER stress-related autophagy in hepatocellular carcinoma. Oncol Rep. 2018;40(4):2206–14.
Yongxi T, Haijun H, Jia** Z, Guoliang S, Hongying P. Autophagy inhibition sensitizes KU-0063794-mediated anti-HepG2 hepatocellular carcinoma cell activity in vitro and in vivo. Biochem Biophys Res Commun. 2015;465(3):494–500.
Zhao Y, Wu H, **ng X, Ma Y, Ji S, Xu X, et al. CD13 induces autophagy to promote hepatocellular carcinoma cell chemoresistance through the P38/Hsp27/CREB/ATG7 pathway. J Pharmacol Exp Ther. 2020;374(3):512–20.
Kaur R, Kanthaje S, Taneja S, Dhiman RK, Chakraborti A. miR-23b-3p modulating cytoprotective autophagy and glutamine addiction in sorafenib resistant HepG2, a hepatocellular carcinoma cell line. Genes. 2022;13(8):1.
Li WY, Li Q, **g L, Wu T, Han LL, Wang Y, et al. P57-mediated autophagy promotes the efficacy of EGFR inhibitors in hepatocellular carcinoma. Liver Int Off J Int Assoc Study Liver. 2019;39(1):147–57.
Chen S, Du Y, Xu B, Li Q, Yang L, Jiang Z, et al. Vaccinia-related kinase 2 blunts sorafenib’s efficacy against hepatocellular carcinoma by disturbing the apoptosis-autophagy balance. Oncogene. 2021;40(19):3378–93.
Zhai B, Hu F, Jiang X, Xu J, Zhao D, Liu B, et al. Inhibition of Akt reverses the acquired resistance to sorafenib by switching protective autophagy to autophagic cell death in hepatocellular carcinoma. Mol Cancer Ther. 2014;13(6):1589–98.
Sun W, Zhang Q, Wu Z, Xue N. miR-101-3p sensitizes hepatocellular carcinoma cells to oxaliplatin by inhibiting Beclin-1-mediated autophagy. Int J Clin Exp Pathol. 2019;12(6):2056–65.
Zou L, Sun P, Zhang L. miR-651-3p enhances the sensitivity of hepatocellular carcinoma to cisplatin via targeting ATG3-mediated cell autophagy. J Oncol. 2021;2021:5391977.
Okubo S, Ohta T, Shoyama Y, Uto T. Steroidal saponins isolated from the rhizome of dioscorea tokoro inhibit cell growth and autophagy in hepatocellular carcinoma cells. Life (Basel, Switzerland). 2021;11(8):1.
Ji Y, Li L, Ma YX, Li WT, Li L, Zhu HZ, et al. Quercetin inhibits growth of hepatocellular carcinoma by apoptosis induction in part via autophagy stimulation in mice. J Nutr Biochem. 2019;69:108–19.
Wei PL, Huang CY, Chang YJ. Propyl gallate inhibits hepatocellular carcinoma cell growth through the induction of ROS and the activation of autophagy. PLoS ONE. 2019;14(1):e0210513.
Yu H, Qiu Y, Pang X, Li J, Wu S, Yin S, et al. Lycorine promotes autophagy and apoptosis via TCRP1/Akt/mTOR axis inactivation in human hepatocellular carcinoma. Mol Cancer Ther. 2017;16(12):2711–23.
Krause GC, Lima KG, Levorse V, Haute GV, Gassen RB, Garcia MC, et al. Exenatide induces autophagy and prevents the cell regrowth in HepG2 cells. EXCLI J. 2019;18:540–8.
Wang M, Huang C, Su Y, Yang C, **a Q, Xu DJ. Astragaloside II sensitizes human hepatocellular carcinoma cells to 5-fluorouracil via suppression of autophagy. J Pharm Pharmacol. 2017;69(6):743–52.
Lai HY, Tsai HH, Yen CJ, Hung LY, Yang CC, Ho CH, et al. Metformin resensitizes sorafenib-resistant HCC cells through AMPK-dependent autophagy activation. Front Cell Dev Biol. 2020;8:596655.
Han R, Li S. Regorafenib delays the proliferation of hepatocellular carcinoma by inducing autophagy. Pharmazie. 2018;73(4):218–22.
Chang WT, Liu W, Chiu YH, Chen BH, Chuang SC, Chen YC, et al. A 4-phenoxyphenol derivative exerts inhibitory effects on human hepatocellular carcinoma cells through regulating autophagy and apoptosis accompanied by downregulating α-tubulin expression. Molecules (Basel, Switzerland). 2017;22(5):1.
Rong LW, Wang RX, Zheng XL, Feng XQ, Zhang L, Zhang L, et al. Combination of wogonin and sorafenib effectively kills human hepatocellular carcinoma cells through apoptosis potentiation and autophagy inhibition. Oncol Lett. 2017;13(6):5028–34.
Yao X, Li X, Zhang D, **e Y, Sun B, Li H, et al. B-cell lymphoma 2 inhibitor ABT-737 induces Beclin1- and reactive oxygen species-dependent autophagy in Adriamycin-resistant human hepatocellular carcinoma cells. Tumour Biol J Int Soc Oncodev Bio Med. 2017;39(3):1010428317695965.
Zhao F, Feng G, Zhu J, Su Z, Guo R, Liu J, et al. 3-Methyladenine-enhanced susceptibility to sorafenib in hepatocellular carcinoma cells by inhibiting autophagy. Anticancer Drugs. 2021;32(4):386–93.
Rasheduzzaman M, Yin H, Park SY. Cardiac glycoside sensitized hepatocellular carcinoma cells to TRAIL via ROS generation, p38MAPK, mitochondrial transition, and autophagy mediation. Mol Carcinog. 2019;58(11):2040–51.
Singh MP, Cho HJ, Kim JT, Baek KE, Lee HG, Kang SC. Morin hydrate reverses cisplatin resistance by impairing PARP1/HMGB1-dependent autophagy in hepatocellular carcinoma. Cancers. 2019;11(7):1.
Zhang N, **e H, Lu W, Li F, Li J, Guo Z. Chloroquine sensitizes hepatocellular carcinoma cells to chemotherapy via blocking autophagy and promoting mitochondrial dysfunction. Int J Clin Exp Pathol. 2017;10(9):10056–65.
Yang Y, Liao Y, Gui YP, Zhao L, Guo LB. GL-V9 reverses adriamycin resistance in hepatocellular carcinoma cells by affecting JNK2-related autophagy. Chin J Nat Med. 2020;18(7):491–9.
Acknowledgements
The authors thank Academic Proofreading (www.academicproofreading.uk) for editing the first draft of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. Research in SCT’s laboratory is supported by the Fundamental Research Grant Scheme of the Ministry of Higher Education, Malaysia (No. FRGS/1/2019/SKK08/UKM/02/9) and the Research University Grant of Universiti Kebangsaan Malaysia (No. GUP-2020-076). The funders have no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the writing of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hashemi, M., Nadafzadeh, N., Imani, M.H. et al. Targeting and regulation of autophagy in hepatocellular carcinoma: revisiting the molecular interactions and mechanisms for new therapy approaches. Cell Commun Signal 21, 32 (2023). https://doi.org/10.1186/s12964-023-01053-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12964-023-01053-z