Abstract
Background
Poor endometrial receptivity is a major factor that leads to recurrent implantation failure. However, the traditional method cannot accurately evaluate endometrial receptivity. Various studies have indicated that microRNAs (miRNAs) are involved in multiple processes of embryo implantation, but the role of miRNAs in endometrial receptivity in patients with recurrent implantation failure (RIF) remains elusive. In the present study, we investigated the presence of pinopodes and the roles of miR-30d-5p, suppressor of cytokine signalling 1 (SOCS1) and the leukaemia inhibitory factor (LIF) pathway in women with a history of RIF during the implantation window.
Methods
Endometrial tissue samples were collected between January 2018 to June 2019 from two groups of women who underwent in vitro fertilisation and embryo transfer (IVF-ET) or frozen ET. The RIF group included 20 women who underwent ≥ 3 ETs, including a total of ≥ 4 good-quality embryos, without pregnancy, whereas the control group included 10 women who had given birth at least once in the past year. An endometrial biopsy was performed during the implantation window (LH + 7). The development of pinopodes in the endometrial biopsy samples from all groups was evaluated using scanning electron microscopy (SEM). Quantitative reverse transcription-polymerase chain reaction and western blotting were used to investigate the expression levels of miR-30d-5p, SOCS1, and the LIF pathway.
Results
The presence of developed pinopodes decreased in patients with RIF on LH + 7. The expression level of miR-30d-5p decreased in the endometria during the implantation window of patients with RIF, whereas the mRNA and protein levels of SOCS1 were significantly higher in the RIF group than in the control group. Furthermore, a negative correlation was observed between the expression of miR-30d-5p and SOCS1 (r2 = 0.8362). In addition, a significant decrease in LIF and p-STAT3 expression was observed during the implantation window in patients with RIF.
Conclusions
MiR-30d-5p and SOCS1 may be potential biomarkers for endometrial receptivity. Changes in pinopode development and abnormal expression of miR-30d-5p, SOCS1 and LIF pathway in the endometrium could be the reasons for implantation failure.
Similar content being viewed by others
Background
Infertility is a global reproductive health problem that affects 10–15% of the reproductive-age couples [1]. After 40 years of development, in vitro fertilization (IVF) and embryo transfer (ET) have become an effective and common therapeutic approaches for couples with infertility [2]. However, in IVF cycles, many couples with infertility fail to achieve pregnancy repeatedly with no signs of embryo implantation or production of HCG. One possible cause of the unsuccessful implantation rate is reduced endometrial receptivity, although the transferred embryos are of high quality [3].
Recurrent implantation failure (RIF) is defined as failure to achieve a clinical pregnancy after transfer of at least four good-quality embryos (score ≥ 7) in a minimum of three fresh or frozen cycles [4]. In some patients with RIF, endometrial receptivity may be impaired, leading to the window of implantation (WOI) being displaced and asynchrony between the functional embryo and endometrium tissue, which may result in implantation failure [5]. Therefore, the assessment of endometrial receptivity is of utmost importance in the IVF procedures to increase the rate of implantation in patients with RIF. During WOI, the endometrium undergoes well-defined morphological and molecular changes that allow embryos to adhere and invade, including the develo** pattern of microvilli of the epithelial cells and pinopode growth, and alterations in various signalling molecules and inflammatory cytokines [6]. Despite advances in the understanding of implantation failure, the pathogenesis of RIF is complex and requires a multidimensional explanation.
Pinopodes are mushroom-like protrusions that appear on the apical surface of endometrial luminal epithelial cells. These protrusions are formed at the beginning of the WOI by cell swelling and the fusing of several adjacent microvilli together, and then reach the maximum size [7]. Pinopodes are the ultrastructural markers of endometrial receptivity, as they are likely to be the preferred sites of embryo–endometrium interactions [8]. Furthermore, the appearance of pinopodes is consistent with the expression of other biomarkers of endometrial receptivity in humans, such as leukaemia inhibitory factor (LIF) and its receptor, integrin αVβ3, mucin 1 [9], and homeo-box gene 10 [10].
MicroRNAs (miRNAs) are endogenous non-coding RNAs that regulate gene expression by post-transcriptional degradation and/or translational repression of target messenger RNAs (mRNAs). Various studies have shown that miRNAs orchestrate a large variety of physiological processes in humans and exert critical effects on factors that affect the embryo implantation process [11]. In humans, efforts have been made to describe the miRNA profiles in isolated endometrial epithelial cells during the late proliferative phase and mid-secretory phase, which suggest a new level of suppression of gene expression during epithelial cell proliferation in the receptive endometrium [12]. In recent studies, miRNA profiles were described in patients undergoing IVF in natural and stimulated cycles, and the expression levels of miRNAs were compared between receptive and pre-receptive endometrium [13]. Interestingly, miR-30d was consistently upregulated during the acquisition of endometrial receptivity compared to the pre-receptive stage. However, no studies have investigated the relationship between miR-30d and classic biomarkers of endometrial receptivity, additionally, the expression of miR-30d in the endometria of patients with RIF remains unclear. In the present study, we evaluated the expression patterns of pinopodes on the endometrial surface, and then we investigated the expression level of miR-30d-5p. SOCS1 and LIF pathway in women with RIF during WOI.
Methods
Patients and tissues
All study subjects in this study were registered at the Reproduction Medical Center, ** analysis, and relevant hormone and immunological tests.
Collection of endometrial biopsies
Follicle development was monitored using transvaginal ultrasound from day 10 of menstruation onwards identification of a dominant follicle ≥ 15 mm, and serum LH and E2 levels were quantified daily. The day on which LH level peaked (≥ 20 mIU/mL) was considered as the day of LH + 0, and the WOI was defined as the day of LH + 7. Endometrial specimens were collected in a cycle before ET cycle using a Pipelle sampler (Prodimed, France) during the implantation period on the 7th day after the LH surge (LH + 7). The obtained endometrial tissues were immediately sent to the laboratory for further processing within 30 min after the endometrial biopsy. One part was examined the presence of pinopodes by scanning electron microscopy, and the other part was stored at -80 °C until subsequent RNA and protein extraction.
Scanning electron microscopy (SEM)
To examine the presence of pinopodes in luminal epithelium, one part of the specimens was fixed in 0.1 M PBS (pH 7.4) containing 2.5% glutaraldehyde overnight, The samples were washed twice in buffer containing sodium cacodylate and calcium chloride (pH 7.4) and once in distilled water. Thereafter, they were dehydrated, first in increasing concentrations of ethanol (70, 95, and 99.5%), and then in acetone, and dried in a critical point drier with carbon dioxide. The specimens were mounted on a specimen holder, coated with platinum, and examined using a JEOL 820 scanning electron microscope (JEOL, Tokyo, Japan). Scanning electron micrographs of pinopodes were obtained from 10 randomly selected areas of each tissue specimen and were graded semiquantitatively. Based on the literature, pinopode coverage of the uterine endometrium can be scored as follows: 0 (absence of pinopodes), 1 (covering < 25% of the surface epithelium, few), 2 (covering 25–50% of the surface epithelium, moderate), or 3 (covering > 50% of the surface epithelium, abundant) [Full size image
Prediction of the interaction between target mRNA and miR-30d-5p
It is known that miRNAs can bind to their target sequences in the 3ʹ-UTR of mRNAs. To verify whether miR-30d-5p can enhance the degradation of a certain mRNA, the bioinformatics software starBase 3.0 was used to search for miR-30d-5p targets. SOCS1 was selected as the predicted target with a high score. miR-30d-5p contains the binding site of SOCS1 fragment (Fig. 2).
Expression of miR-30d-5p and SOCS1 in fertile women and patients with RIF
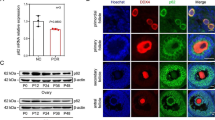
Decreased expression of miR-30d-5p was detected in the RIF group, when compared with the control group (p = 0.0049, Fig. 3A). In contrast, the mRNA and protein expression of SOCS1 was higher in the RIF group than in the control group (p = 0.0005, p = 0.0075, Fig. 3B, C). Spearman correlation analysis showed a negative correlation between the expression of miR-30d-5p and SOCS1 at the RNA level (r2 = 0.8362, p < 0.0001) (Fig. 3D).
Comparison of miR-30d-5p and SOCS1 expression in endometrium samples from the RIF and control groups. A, B The relative expression levels of miR-30d-5p and SOCS1 in the RIF group (n = 20) and the control group (n = 10), as detected via RT-qPCR; U6 was used as an internal control for miR-30d-5p; GAPDH was used as an internal control for SOCS1. C The relative expression level of SOCS1 protein in the RIF group (n = 20) and the control group (n = 10), as detected via western blotting; GAPDH was used as internal control. D Spearman correlation suggests a significant negative correlation between the expressions of miR-30d-5p and SOCS1. The Spearman correlation coefficient, P values, and sample numbers are indicated on the upper right of the plot. *P < 0.05
Expression of LIF, STAT3 and p-STAT3 in fertile women and patients with RIF
The mRNA levels of LIF were detected in endometrium by RT-qPCR. Data showed that the mRNA levels of LIF were lower in the RIF group than that in the control group (p < 0.0001; Fig. 4A). The protein levels of LIF and p-STAT3 were lower in the RIF group than that in the control group (p = 0.0002, p = 0.0038; Fig. 4B, C and D), and the expression of STAT3 proteins showed no difference between the two groups.
Comparison of miR-30d-5p and SOCS1 expression in endometrium samples from the RIF and control groups. A The relative expression level of LIF mRNA in the RIF group (n = 20) and the control group (n = 10), as detected via RT-qPCR; GAPDH was used as an internal control for LIF. B The relative expression levels of LIF, p-STAT3 and STAT3 protein in the RIF group (n = 20) and the control group (n = 10), as detected via western blotting; GAPDH was used as internal control. C, D Data of expression of LIF and p-STAT3 protein were expressed as mean ± SD. *P < 0.05